Translate this page into:
An observational study to determine the sensitizing potential of orthopedic implants
Corresponding author: Dr. Sanjeev Handa, Department of Dermatology Venereology and Leprology, Postgraduate Institute of Medical Education and Research, Chandigarh - 160 012, India. handa_sanjeev@yahoo.com
-
Received: ,
Accepted: ,
How to cite this article: Shanmugham HA, Handa S, De D, Dhillon MS, Aggarwal S. An observational study to determine the sensitizing potential of orthopedic implants. Indian J Dermatol Venereol Leprol 2021;87:826-30.
Abstract
Introduction:
Patients who receive orthopedic implants have been shown to develop sensitivity to its components and there are concerns that this sensitivity might lead to contact dermatitis or implant-related problems like loosening and/or failure. The objective of the study was to determine the sensitizing potential of orthopedic implants.
Methods:
Fifty-four patients undergoing knee, hip, or shoulder replacement surgeries between July 2014 and July 2015 were recruited. Patch tests were performed before the implant surgery with 10 allergens likely to be implicated in metal hypersensitivity. Postimplant patch test was performed 6 months after surgery. A majority of the patch tests were applied on the arms.
Results:
Four positive reactions were recorded in the preimplant patch tests – three positive reactions to nickel and one to chromium. Thirty patients made themselves available for the follow-up patch test. The incidence of new contact sensitivity to components of implants was 13.8% (4/29) at 6 months. One patient who had undergone knee replacement developed eczematous lesions around the knee joint after surgery. This patient tested negative to patch test at both the times.
Limitations:
Short follow-up duration and performing patch tests on the arms, a site known to elicit less positive patch test response compared to the back in sensitized individuals, are limitations of the study. Conclusion: There is an increase in the sensitivity to implanted components after 6 months of joint replacement surgery. The incidence of new sensitivity to a component of the implant was 13.8% (4/29). In this context, nickel is a good sensitizer and could sensitize 50% of patients who received a nickel-containing implant.
Keywords
Cobalt
contact sensitization
nickel
orthopedic implants
Introduction
Total joint replacements, especially of the knee and hip, are increasingly being done in recent times. Traditionally, stainless steel implants were used; however, cobalt–chromium alloys and titanium–aluminum alloys are preferred currently.1 There are concerns regarding the possibility of corrosion, thereby liberating metal ions, which may act as haptens and induce sensitivity in the recipients. Reactions to metal implants may manifest as impaired wound healing, chronic pain or swelling around the joint, dermatitis, or implant loosening.2
Among metals, hypersensitivity is most common to nickel, chromium, and cobalt. Incidentally, these are also the metals most commonly used in orthopedic implants. In studies conducted by Granchi et al., nickel was the most common metal sensitizer in patients undergoing joint replacements followed by cobalt, chromium, and manganese.3,4 Sensitivity to titanium, vanadium, and polymethyl methacrylate bone cement has also been rarely reported.5,6 A recent review by Mitchelson et al. found that sensitivity to implant components had increased from 9.1% preoperatively to 14.1% postoperatively.7
We recruited patients who planned to receive orthopedic metal implants and performed patch testing before the implant surgery and 6 months after it. It was aimed at determining the sensitizing potential of orthopedic implants and translation of this sensitization into clinically manifest contact dermatitis, if any.
Methods
This prospective study was carried out in the contact dermatitis clinic of the Department of Dermatology, Postgraduate Institute of Medical Education and Research, Chandigarh. Ethics Committee approval was obtained before study initiation. Fifty-four consenting patients undergoing knee and/or hip or shoulder replacement surgeries between July 2014 and July 2015 were recruited.
Exclusion criteria
Patients who already had an orthopedic implant in situ
Patients who were on oral steroid dose equivalent to or higher than 15 mg of prednisolone
Patients who were on chemotherapy or immunosuppressive therapy
Patients who had dermatitis at the test site and were therefore unfit for patch test.
At the first visit, a written informed consent was taken and the baseline demographic characteristics of the patients were noted. Detailed history including presence of other bone or joint implants and history of previous allergy to metals in cosmetic jewelry, belts, buckles, watches, or dental implants and other metal ornaments was recorded. A baseline dermatologic examination was carried out. Before the implant surgery, patch test was performed on all patients with the preimplant series of 10 allergens likely to be implicated in metal hypersensitivity following standard patch test protocol and precautions. The details of the allergens used are mentioned in Table 1. Readings were taken at 48 and 96 hours. Wherever possible, a delayed reading was taken at 168 hours to note for delayed reactions. The results for each test site were recorded according to International Contact Dermatitis Research Group criteria.8 The patch test to a particular allergen was considered positive when reading at 96 hours and/or 168 hours was recorded as 1+ or stronger.
| Allergen (with concentration percentage w/w and vehicle) |
|---|
| Nickel sulfate hexahydrate 5% pet |
| Potassium dichromate 0.5% pet |
| Cobalt chloride hexahydrate 1% pet |
| Titanium dioxide 10% pet |
| Vanadium 5% pet |
| Methyl methacrylate 2% pet |
| N, N-Dimethyl-4-toluidine 5% pet |
| Hydroquinone 1% pet |
| Benzoyl peroxide 1% pet |
| Gentamicin sulfate 20% pet |
Aq: aqueous, pet: petrolatum
All the patients received the orthopedic implant planned for their condition, irrespective of the results of their patch tests. The patients were asked to report back for a postimplant patch test, if they developed any symptom or sign of dermatitis either on the skin overlying the implant or at remote sites, or after 6 months of implant, whichever was earlier. A positive patch test to a certain allergen was considered relevant if that substance was a component of the alloy that was implanted. Allergens manufactured by Chemotechnique Diagnostics, Malmo, Sweden® were used for the tests.
Statistical analysis was performed using Fisher’s exact test. All statistical tests were two-sided and were performed at a significance level of α = 0.05. Statistical analysis was carried out using Statistical Package for Social Sciences (SPSS Inc., Chicago, IL, USA; version 21.0 for Windows).
Results
The flow of the study is presented in Figure 1. The demographic and clinical characteristics of the patients are presented in Table 2.
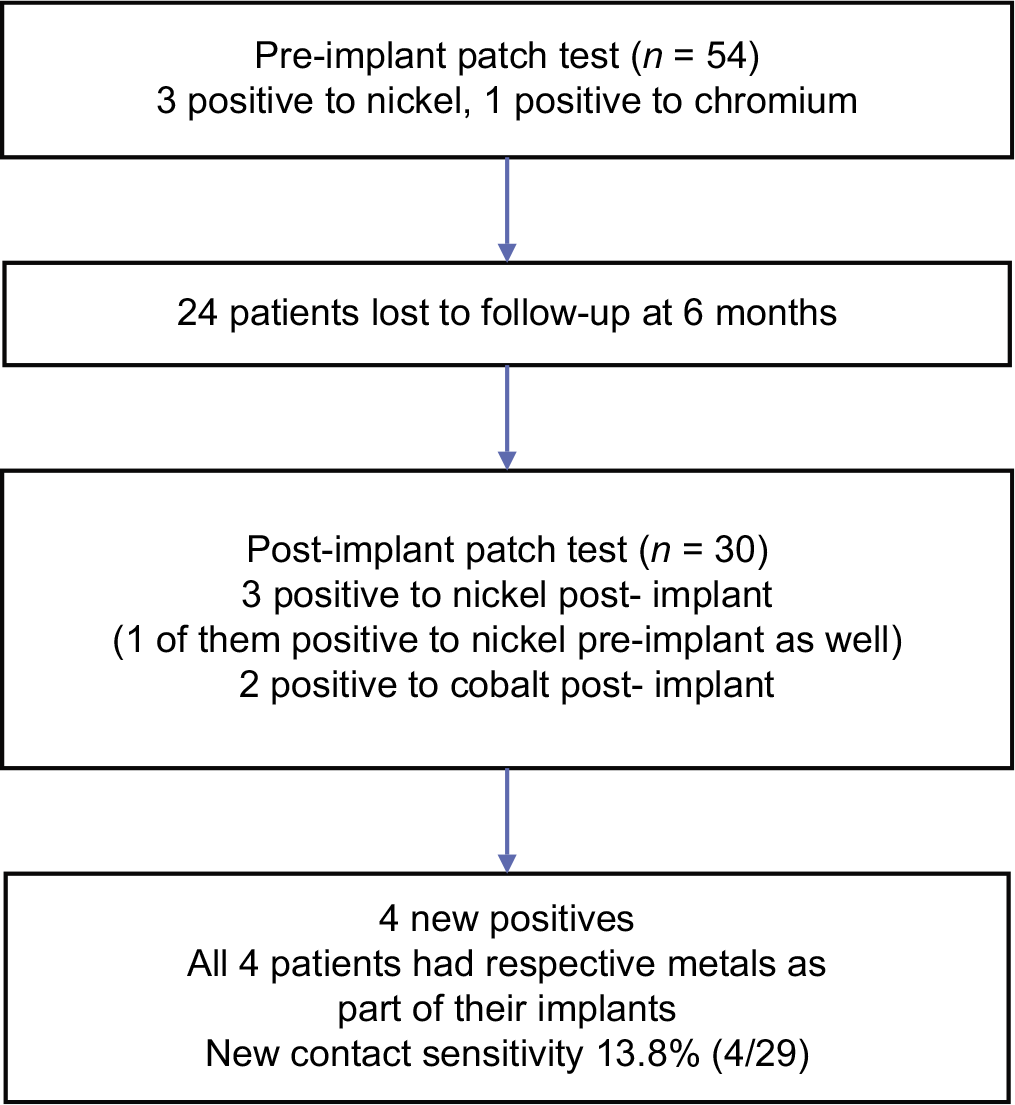
- Flow of the study
| Characteristics | Value |
|---|---|
| Number of patients | 30 |
| Males: females | 16:14 |
| Age (years), mean±SD | 55.0±13.7 |
| History of atopy (%) | 2 (6.7) |
| History of hand eczema (%) | 3 (10) |
| History of clinical metal allergy (%) | 6 (20) |
| Presence of metal dental fillings in situ (%) | 6 (20) |
SD: standard deviation
Preimplant patch test results
The results are presented in Table 3. There were three positive reactions to nickel and one to chromium [Figure 2]. The percentage of positivity to nickel was more (22.2%) common in those with a clinical history of metal allergy compared with those without a history (2.3%) (Fisher’s exact test, P = 0.07). There were two irritant reactions, one each to chromium and methyl methacrylate.
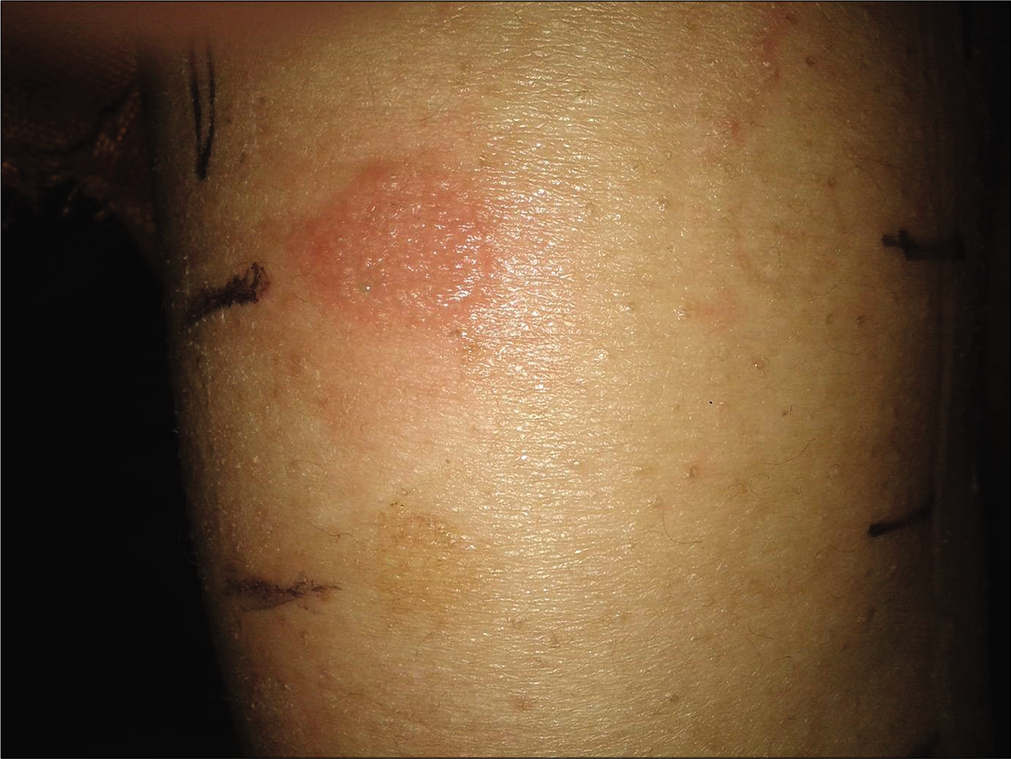
- Preimplant positive (2+) reaction to nickel in a female patient who had history of itching and oozing while using artificial jewelry
| Allergen | Frequency of positive reactions, n (%) | Correlation with history of metal allergy |
|---|---|---|
| Preimplant (n=54) | ||
| Nickel sulfate | 3 (5.5) | Of the 3 patients, 2 had history of metal allergy |
| Potassium dichromate | 1 (1.9) | - |
| Total | 4 (7.4) | Of the 4 patients, 2 had history of metal allergy |
| Postimplant (n=30) | ||
| Nickel sulfate | 3 (10) | Two were new reactions. All 3 patients had received nickel as part of the implant (2 relevant new reactions) |
| Cobalt chloride | 2 (6.7) | Both patients had received cobalt as part of the implant (2 relevant new reactions) |
Characteristics of postimplant test group
At 6 months of follow-up, 30 of the 54 patients recruited made themselves available for postimplant patch test. Two patients with a positive reaction to nickel and one patient with a positive reaction to chromium at baseline were among those who did not return. In the postimplant test group, only one patient had history of eczema, and it was present even before the implant surgery was performed, that is, there was no incident case of dermatitis.
Postimplant patch test results
The results are presented in Table 3 and Figure 3. A few new positive patch test reactions were observed after implant.
Among these, only nickel and cobalt were present in the implants used in the patients. None of these patients had undergone metal dental filling during the study which could be the other possible reason of new patch test reaction. There were five irritant reactions – three to cobalt and one each to nickel and dimethyl toluidine.
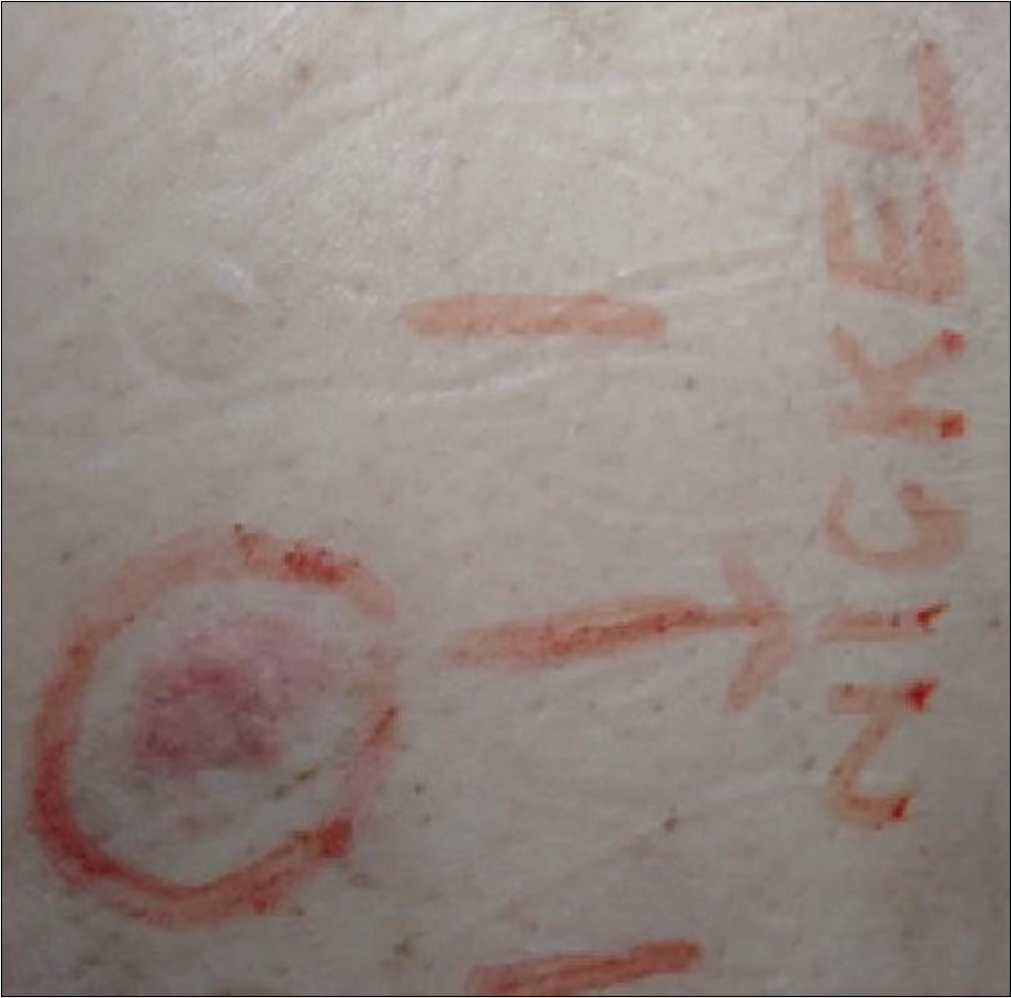
- Postimplant positive reaction (1+) to nickel with erythema and infiltration at 96 hours. The patient tested negative to nickel in the preimplant test
Comparison of test results, pre and post implant
Among the 30 patients who were patch tested both at baseline and after 6 months, only one patient had preimplant patch test positivity (to nickel) and five patients had postimplant positivity (three to nickel and two to cobalt). The difference in proportion was not statistically significant (Fisher’s exact test, P = 0.16).
The incidence of new (relevant) contact sensitivity to components of implants was 13.8% (4/29). Nickel was found to be a statistically significant sensitizer (Fisher’s exact test, P = 0.01), and cobalt was found to be a poor sensitizer in this setting. Only 2 of 27 patients who received cobalt-containing implants elicited positive patch test reaction to cobalt (Fisher’s exact test, P = 1).
Incident eczema
Among the 30 patients who completed 6 months of follow-up, one patient developed incident eczema. The patient did not elicit patch test reaction to any of the tested allergens.
Discussion
Hypersensitivity to metals and metallic substances is a common finding in the general population. The most common sensitizer has traditionally been nickel and its sensitivity is more common in females.9 In a series of 1000 patients, sensitivity to nickel was observed in about 13%.10 In our cohort, as expected, nickel was the most common sensitizer. The baseline sensitivity to nickel was 5.6% (3/54) and all these patients were females. The sensitivity to nickel had increased to 10% (3/30) among patients who completed 6 months of follow-up. The subjects were elderly people, who were admitted with a knee or hip or shoulder problem, and they had to remain supine, thus excluding the option of putting the patches on the back. As a consequence, most of the patients were tested on the arm, which is known to be less sensitive than the back as a site for patch testing.11 This probably explains the lesser number of positive reactions observed by us.
Among nonsensitized patients who received a nickel- containing implant, the rate of conversion to positivity was 50% (2/4). Although nickel is a good sensitizer in the general population from sources outside the body, it has been unclear as to how much sensitivity nickel can induce after liberation from implants. Our findings show that nickel can cause sensitization, when it is part of an implanted material.
Two patients developed new sensitivity to cobalt and they had both received cobalt as part of their implants. The rate of conversion to positivity among nonsensitized patients who received a cobalt containing implant was 7.4% (2/27).
In the recruited patients, the baseline hypersensitivity to metals was 7.6% (n = 4). Of the four patients who tested positive for a metal at baseline, only two of them (50%) gave history of metal allergy. This makes preimplant testing of patients important, as this will unmask patients who are already sensitized with these components and help us take a considered decision on which type of implant to use.
There are differences between patients with preimplant vs. postimplant patch test positivity. One patient in our study who had a positive reaction to nickel before implant received an implant containing the same metal. Although no dermatitis was noticed in the patient at 6 months of follow-up, it is prudent that such patients be followed up for a longer period of time, because they would be expected to have a higher chance of developing allergic reactions to nickel present in the implanted material.
One of the prime concerns for the dermatologist when a patient with history of metal allergy is referred before an implant surgery is to decide whether to perform a patch test or not. A patch test would be useful if a positive result could predict the possibility of developing allergic reactions, either in the form of contact dermatitis or as a contributory factor to implant failure. Analyses have shown that patch testing cannot reliably predict this risk.3,12 Until long-term prospective studies with adequate follow-up are performed, it would be difficult to recommend the routine use of patch test before implant surgeries. However, common sense dictates that any patient with a history of metal allergy should be tested with the metals that the patient is planned to receive. It is relatively easy to advice patients who test negative for the components of the planned implant. In patients with a positive reaction to metals that are present in the proposed implant, planning the future course of management is complicated. In a case series by Atanaskova Mesinkovska et al., 21 patients with a history of metal allergy were given allergen-free implants. None developed dermatitis or implant loosening at a mean follow-up of 12 months.13 It would be easy to suggest implants made of titanium, a widely accepted nonsensitizer, for patients with positive patch test reactions to nickel, chromium and cobalt. However, this might not be accepted by all, as this leads to increased cost. Moreover, there are long-term studies with up to 12 years of follow-up which have shown no increase in implant failure in patients with implant components that they are allergic to.14,15 Therefore, with the data available, it would probably be best left to the surgeon and the patient to decide which course to follow.
Limitations
The follow-up period of 6 months may be insufficient to detect development of new contact sensitivities to the implant components. Also, a majority of patch tests were performed on the arm, a site which is known to be less sensitive than the back for patch testing.
Conclusion
After 6 months of hip/knee replacement surgery, the sensitivity to implanted metals had risen from baseline. Among the patients, around one-seventh developed new sensitivity to a component of the implant they had received without occurrence of new contact dermatitis in them. Nickel can sensitize almost half the patients who receive a nickel-containing implant possibly having implications for them in their postimplant life.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patients have given their consent for their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Cutaneous and systemic hypersensitivity reactions to metallic implants. Dermatitis. 2011;22:65-79.
- [CrossRef] [PubMed] [Google Scholar]
- Intolerance reactions to knee arthroplasty in patients with nickel/cobalt allergy and disappearance of symptoms after revision surgery with titanium-based endoprostheses. J Dtsch Dermatol Ges. 2009;7:410-3.
- [CrossRef] [PubMed] [Google Scholar]
- Sensitivity to implant materials in patients with total knee arthroplasties. Biomaterials. 2008;29:1494-500.
- [CrossRef] [PubMed] [Google Scholar]
- Sensitivity to implant materials in patients undergoing total hip replacement. J Biomed Mater Res B Appl Biomater. 2006;77:257-64.
- [CrossRef] [PubMed] [Google Scholar]
- Sensitivity to titanium. A cause of implant failure? J Bone Joint Surg Br. 1991;73:25-8.
- [CrossRef] [PubMed] [Google Scholar]
- Lymphocyte response to polymethylmethacrylate in loose total hip prostheses. J Bone Joint Surg Br. 1992;74:825-30.
- [CrossRef] [PubMed] [Google Scholar]
- Biomaterial hypersensitivity: Is it real? Supportive evidence and approach considerations for metal allergic patients following total knee arthroplasty. Biomed Res Int. 2015;2015:137287.
- [CrossRef] [PubMed] [Google Scholar]
- Patch test results from a contact dermatitis clinic in North India. Indian J Dermatol Venereol Leprol. 2011;77:194-6.
- [CrossRef] [PubMed] [Google Scholar]
- Patch testing experience with 1000 patients. Indian J Dermatol Venereol Leprol. 2007;73:313-8.
- [CrossRef] [PubMed] [Google Scholar]
- Patch test methods II Regional variations of patch test responses. Acta Derm Venereol. 1965;45:257-61.
- [Google Scholar]
- Metal hypersensitivity testing in patients undergoing joint replacement: A systematic review. J Bone Joint Surg Br. 2012;94:1126-34.
- [CrossRef] [PubMed] [Google Scholar]
- The effect of patch testing on surgical practices and outcomes in orthopedic patients with metal implants. Arch Dermatol. 2012;148:687-93.
- [CrossRef] [PubMed] [Google Scholar]
- Implantation of orthopaedic devices in patients with metal allergy. Acta Derm Venereol. 1989;69:62-6.
- [Google Scholar]
- The association between metal allergy, total hip arthroplasty, and revision. Acta Orthop. 2009;80:646-52.
- [CrossRef] [PubMed] [Google Scholar]






