Translate this page into:
Anti-P 200 pemphigoid – The most common floor binding subepidermal autoimmune bullous disease in a tertiary care center in south India
Corresponding author: Dr. J. Bede Anand, Department of Dermatology, PSG Institute of Medical Sciences and Research, Coimbatore, Tamil Nadu, India. boy.bede@gmail.com
-
Received: ,
Accepted: ,
Read UPDATED-ARTICLE associated with this - 10.25259/IJDVL_79_20_ER
How to cite this article: Rai R, Anand JB, Shanmugasekar C, Arunprasath P, Chaitra V, Zillikens D, et al. Anti-P 200 pemphigoid – The most common floor binding subepidermal autoimmune bullous disease in a tertiary care center in South India. Indian J Dermatol Venereol Leprol 2021;87:787-91.
Abstract
Background:
The pemphigoid group of diseases may present clinically and immunologically in a very similar fashion. Indirect immunofluorescence microscopy with readily available salt-split human skin in a BIOCHIP™ helps to classify these conditions as those with either with roof binding or floor binding of immunoreactants. Epidermolysis bullosa acquisita, anti-laminin 332 pemphigoid and anti-p200 pemphigoid show floor binding, while in the most frequent type of pemphigoid disease, bullous pemphigoid, epidermal side staining pattern is seen on salt-split skin
Aims:
The aim of the study was to detect the target antigens in sub-epidermal bullous diseases.
Methods:
Forty patients with bullous pemphigoid diagnosed by lesional histopathology and direct immunofluorescence microscopy were re-evaluated by a BIOCHIP™ mosaic containing both tissue substrates and recombinant target antigens. Sera with floor pattern staining on salt-split skin were further evaluated by immunoblotting with dermal extract.
Results:
Five patients with floor staining had anti-p200 pemphigoid.
Limitations:
We could not perform serration pattern analysis of direct immunofluorescence in our patients.
Conclusion:
Histopathology and direct immunofluorescence microscopy cannot differentiate between various entities of pemphigoid diseases. A multivariant approach using a BIOCHIP™ mosaic including salt-split skin followed by immunoblotting with dermal extract helps to identify the target antigen.
Keywords
Anti-p200 pemphigoid
biochip
floor binding
immunoblotting
Introduction
The pemphigoid diseases are a group of immunobullous disorders characterized by autoantibodies against hemidesmosomal structural proteins of the dermal-epidermal junction. The binding of autoantibodies in pemphigoid diseases finally results in separation of the epidermis from the dermis. Clinically, these diseases present with tense blisters, erosions and crusting with or without mucous membrane involvement.1 Histopathology of lesions shows subepidermal bullae with mixed inflammatory infiltrate composed of neutrophils, lymphocytes and eosinophils in the papillary dermis. Direct immunofluorescence microscopy of a perilesional skin biopsy shows linear IgG/IgA, C3 staining in all pemphigoid diseases.2 Indirect immunofluorescence microscopy on salt-split skin further helps to delineate the conditions into roof and floor pattern staining. Bullous pemphigoid is the prototype for roof-binding diseases, while floor pattern binding is seen in epidermolysis bullosa acquisita,3 anti-laminin 332 pemphigoid and anti-p200 pemphigoid.4-8
Aim
The aim of this study was to further differentiate immunologically 40 patients presenting clinically and by direct IF microscopy as bullous pemphigoid in a stepwise manner using indirect immunofluorescence microscopy on a BIOCHIP™ mosaic and immunoblotting with dermal extract. This approach resulted in the diagnosis of anti-p200 pemphigoid in five of these 40 patients.
Methods
After obtaining Institutional Ethical Committee Review Board approval a retrospective cross-sectional study was conducted in PSG Institute of Medical Sciences and Research, Coimbatore of patients seen between July 2018 and September 2019. Forty cases which had presented clinically with tense bulla compatible with pemphigoid group were included in the study for which biopsy and direct immunofluorescence had been done. Lesional histopathology and direct immunofluorescence microscopy were compatible with bullous pemphigoid. They were further probed with BIOCHIP™ mosaic (Dermatology mosaic 7, Euroimmun, Lübeck, Germany) which has miniature incubation fields and includes sections of monkey esophagus and salt-split human skin, recombinant BP180 NC16A and HEK 293 cells expressing recombinant desmoglein 1, desmoglein 3 and the C-terminal domain of BP230.9 Subsequently, sera of patients with floor pattern staining were further evaluated by immunoblotting with dermal extract and the recombinant C-terminus of laminin ϒ1 (University of Lübeck, Germany).6,10,11 Cases with flaccid bullae, erosions and oral ulcers compatible with pemphigus group of disorders were excluded from the study.
Results
Forty cases with subepidermal bullae by lesional histopathology and linear deposits of IgG and/or C3 at the dermal-epidermal junction by direct immunofluorescence microscopy suggestive of bullous pemphigoid were studied [Figure 1]. The BIOCHP™ mosaic confirmed the diagnosis of bullous pemphigoid in 35 cases, while five cases showed floor pattern staining on salt-split skin [Figure 2] with absent autoantibodies against all other antigens present on the employed Biochip mosaic. Immunoblotting with dermal extract and recombinant C-terminus of laminin ϒ1 revealed IgG4 reactivity against both, a 200 kDa protein in all five cases [Figure 3] diagnosing them as anti-p200 pemphigoid. These five anti-p200 pemphigoid cases had a mean age of 56.6 years (range 42–64), four patients were males and one was female. The clinical profiles of these five patients are summarized in Table 1. Three patients (nos. 7,12,20) presented with tense hemmorhagic blisters over thigh and dorsal aspect of penis [Figure 4], tense blisters over dorsal aspect of hands [Figure 5] and erosions over medial aspect of right knee [Figure 6]. The mean age of the 35 BP cases was 65.4 years (range, 58–71); 19 patients were males and 16 were females. The clinical profiles of these 35 patients are summarized in Table 2.
| S. No | Age (years) | Sex | Type of blisters | Areas of blistering | Oral erosions |
|---|---|---|---|---|---|
| 1 | 42 | Male | Tense, clear | Dorsum of hands, feet | - |
| 2 | 50 | Male | Tense, clear and haemorrhagic | Generalized | + |
| 3 | 63 | Female | Tense, clear | Shoulder, chest, back | - |
| 4 | 64 | Male | Tense, clear | Upper arm, shoulder, back | - |
| 5 | 64 | Male | Tense, clear | Back | - |
| S. No | Number of patients | Mean age (years±SD) | Sex (F/M) | Type of blisters | Areas of blistering | Oral erosions |
|---|---|---|---|---|---|---|
| 1 | 23 | 60±5.8 | 11/12 | Tense, clear | Generalized | - |
| 2 | 7 | 58.4±4.2 | 3/4 | Tense, clear | Extremities, dorsum of hands | - |
| 3 | 5 | 59.4±3 | 2/3 | Tense, clear | Trunk, face , scalp | - |
SD: Standard deviation, F: Female, M: Male
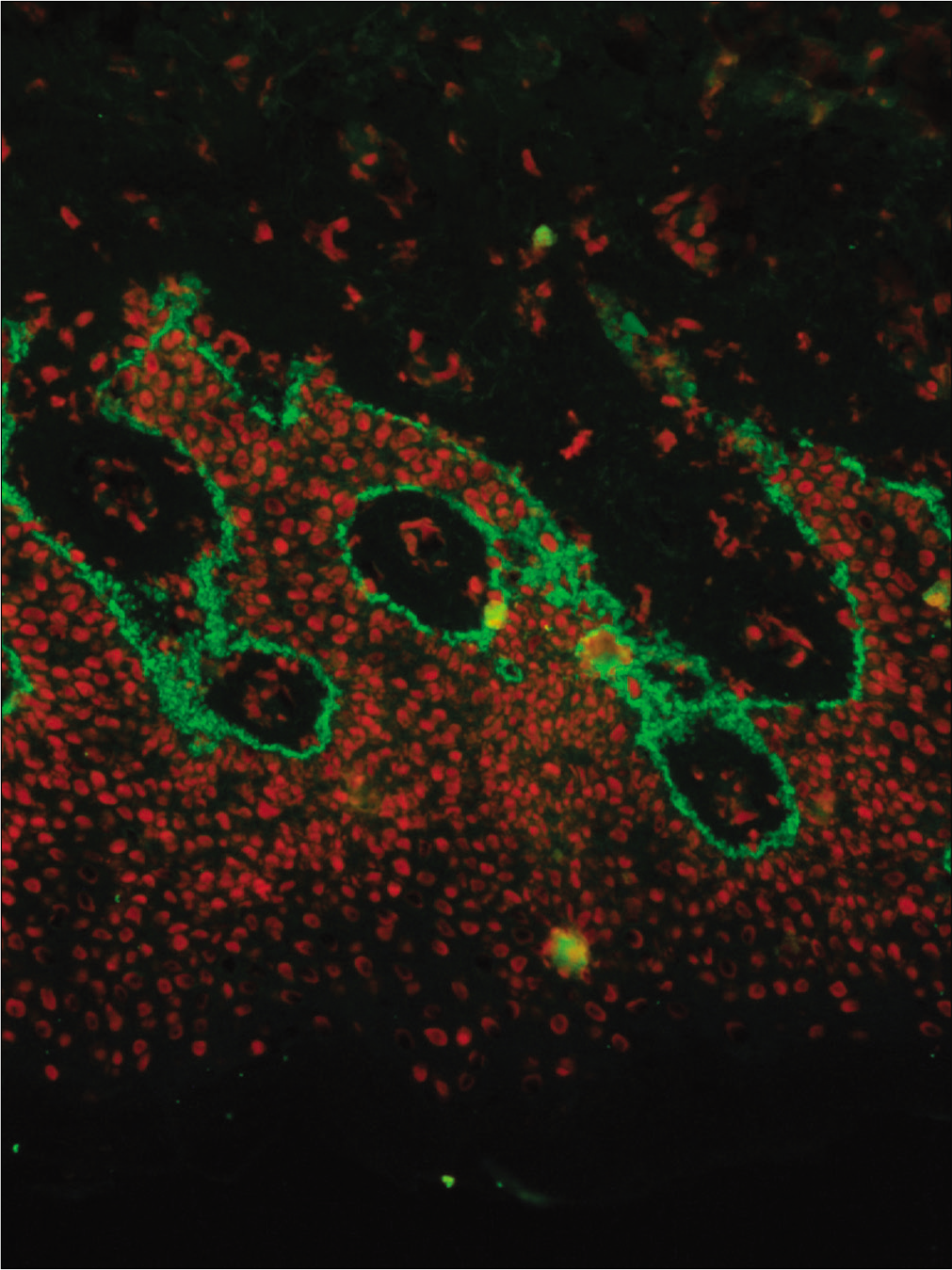
- Direct immunofluorescence of a perilesional biopsy with strong linear staining of C3 at the dermal-epidermal junction in patient No 7 (fluorescein isothiocyanate, 400×)
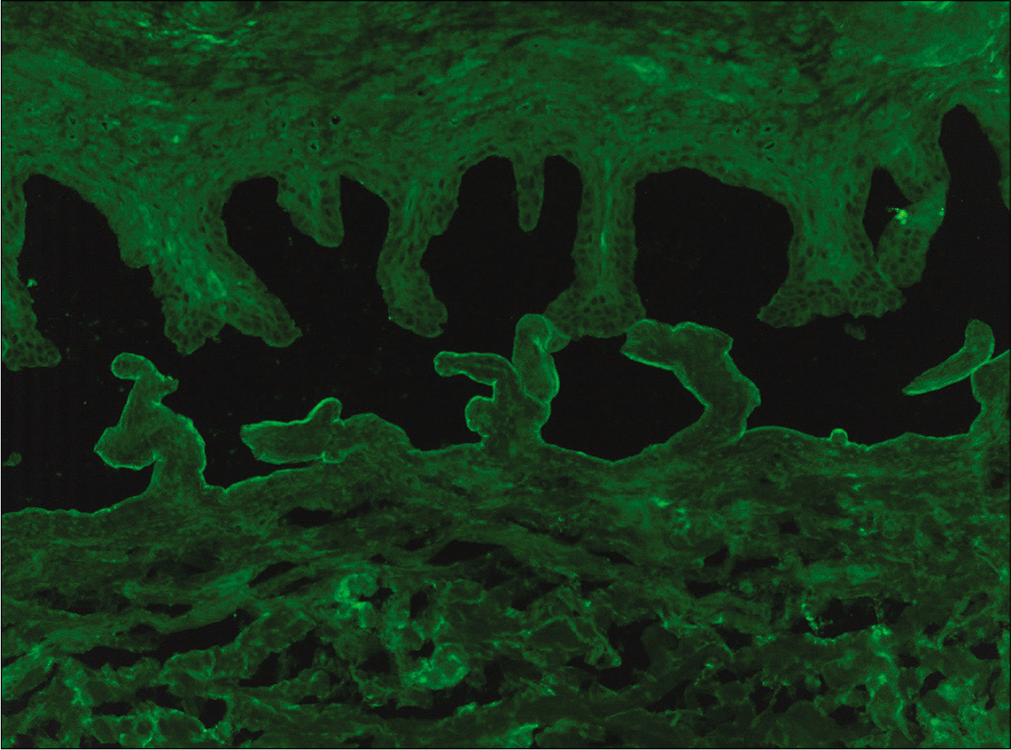
- Binding of IgG along the floor of the artificial split by indirect immunofluorescence microscopy on salt-split skin in patient No 12 (fluorescein isothiocyanate, 200×)
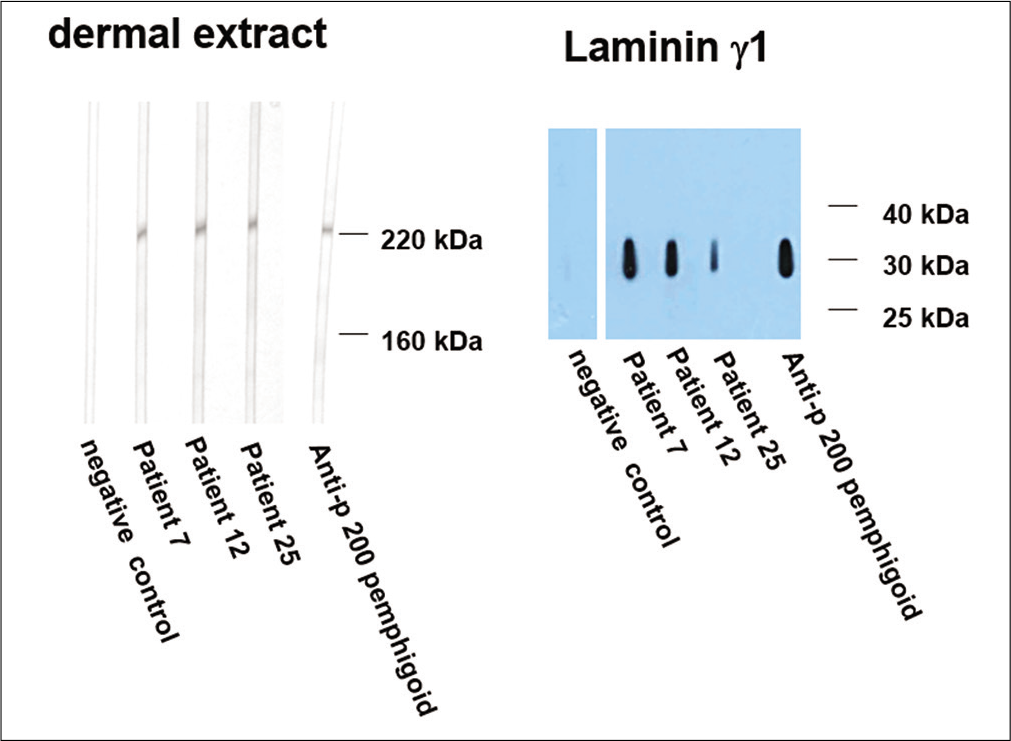
- Immunoblotting of sera with dermal extract (left panel). Sera from patients 7, 12 and 25 show reactivity with a 200 kDa molecule. Western blotting with recombinant laminin ϒ1 (right panel). Sera from patients 7, 12 and 25, like the control anti-p 200 pemphigoid serum, react with this recombinant protein. Negative control sera show no reactivity in both panels
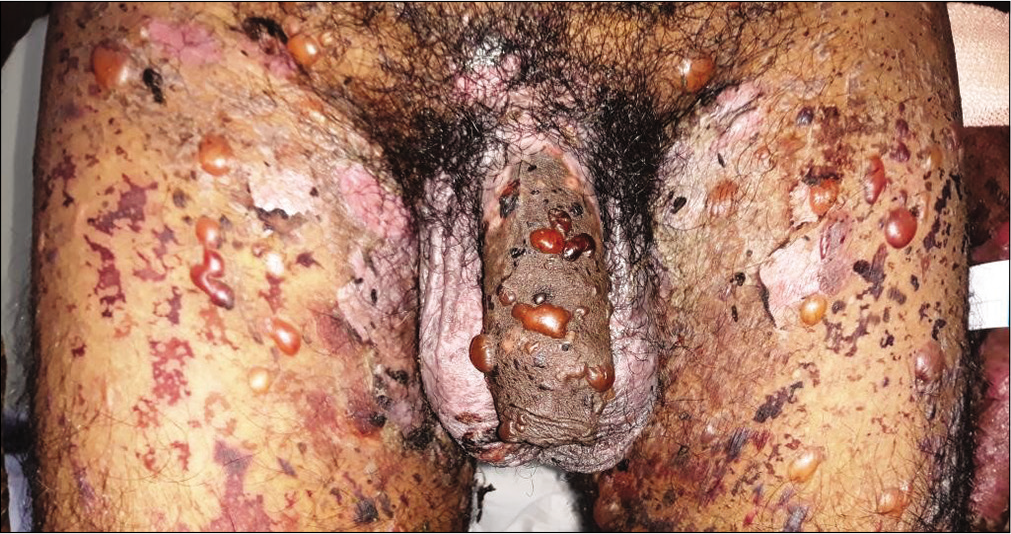
- Tense blisters and erosions on the genitalia and thighs in patient No 7
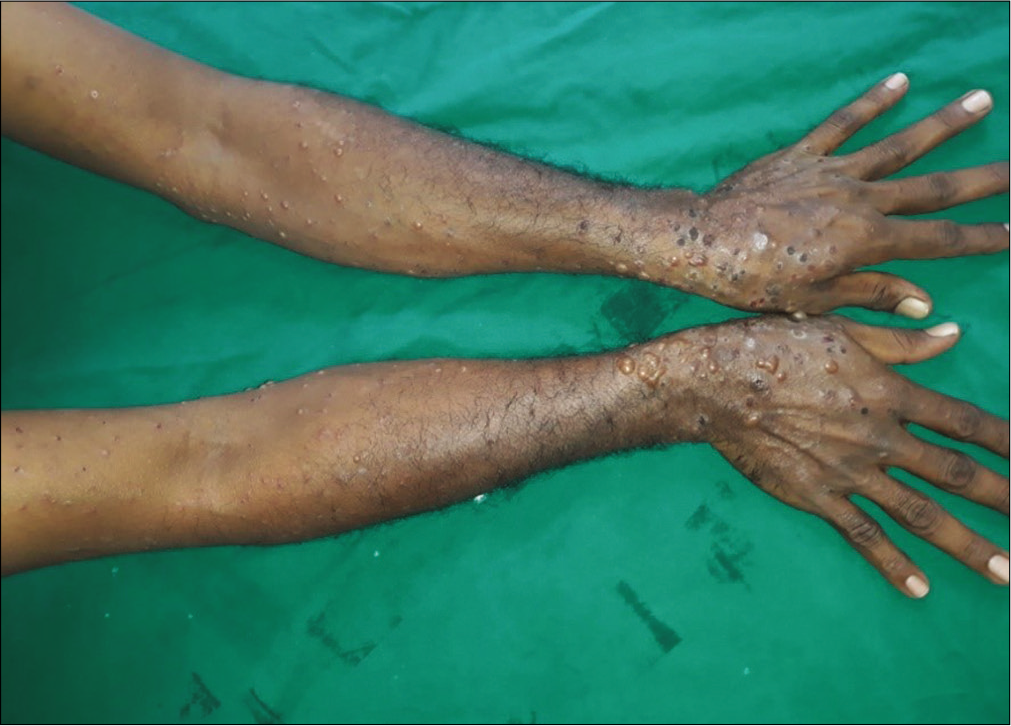
- Numerous tense blisters and erosions on lower arms in patient No 12
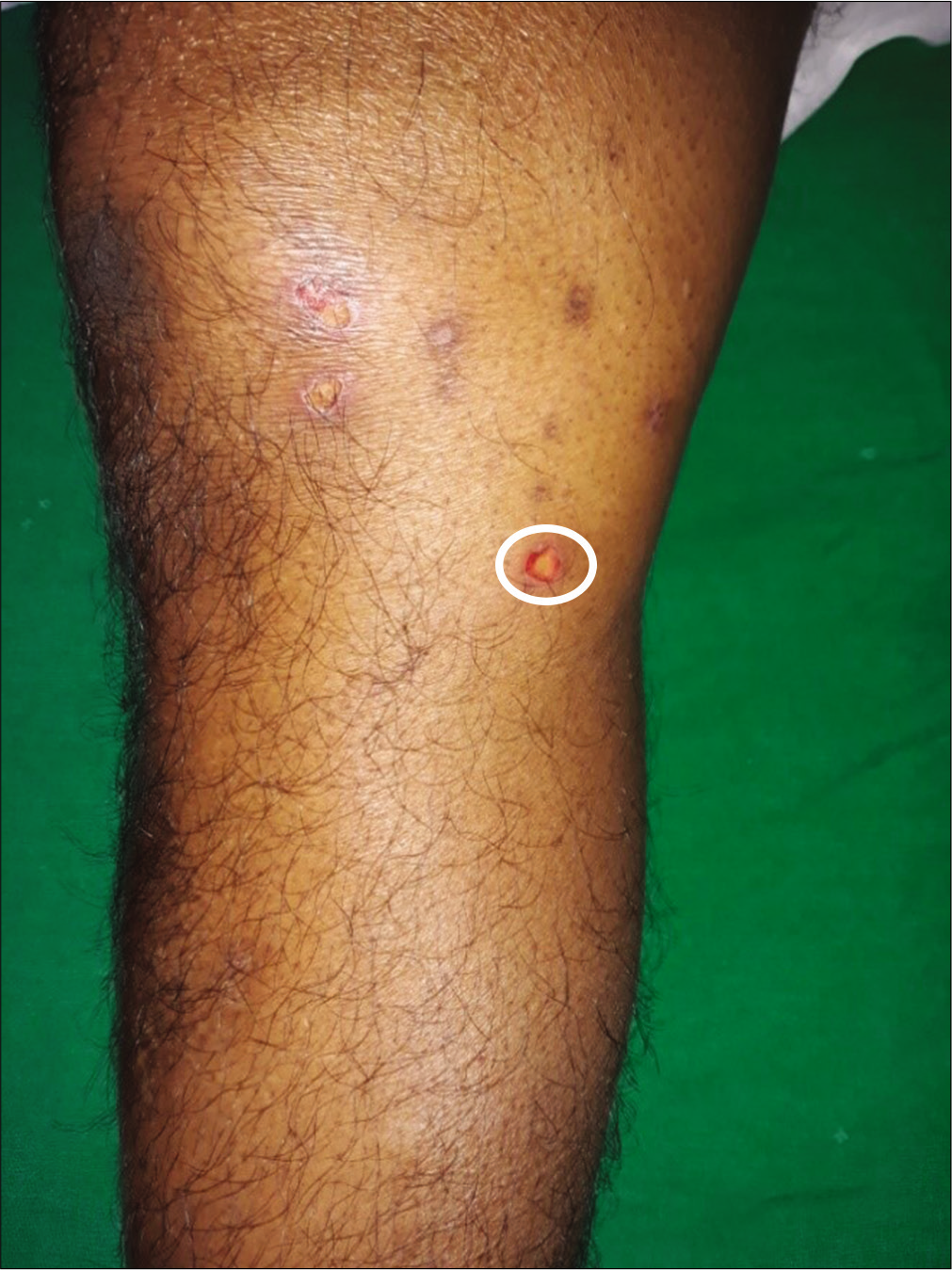
- Erosions on right knee in patient No 20
Discussion
Pemphigoid diseases overlap clinically and it is difficult to differentiate among them based on the clinical presentation alone. All pemphigoid diseases present with tense blisters and erosions with or without mucous membrane involvement. A positive direct immunofluorescence microscopy is the gold standard for the diagnosis of pemphigoid disorders. However, this analysis does not allow the differentiation between the different pemphigoid diseases, except for epidermolysis bullosa acquisita. The latter shows a u-serrated pattern, in contrast to all other pemphigoid diseases demonstrating an n-serrated pattern.12,13 This technique with its high recognition rate can be implemented for routine diagnosis of pemphigoid diseases.12
Hence, serology is required for the exact diagnosis of pemphigoid diseases. A widely used screening test for this is salt-split human skin which shows binding of IgG and/or IgA antibodies to the roof of the artificial blister (in sera with autoantibodies against BP180, BP230 and integrin) or the blister floor (in sera with reactivity against laminin 332, Type VII collagen and the anti-p200 protein).
Epidermolysis bullosa acquisita, a prototype of a floor-binding disease, is a rare pemphigoid disorder with autoantibodies against the non-collagenous amino-terminal domain of Type VII collagen14,15 Two clinical subtypes of epidermolysis bullosa acquisita are recognized; the classical mechanobullous variant resembling hereditary dystrophic epidermolysis bullosa and inflammatory variant of epidermolysis bullosa acquisita that mimics bullous pemphigoid.16 The classical variant is characterized by skin fragility, erosions, blisters, crusts and scars on trauma-prone areas such as the hands, knuckles, elbows, knees and toes. The inflammatory variant manifests as tense blisters on erythematous base. Clinically, it is not possible to distinguish the inflammatory variant from most other pemphigoid diseases.16-18 Epidermolysis bullosa acquisita has a poorer prognosis than most other pemphigoid diseases and it is more difficult to treat. Hence, it is important to distinguish between these disorders. Enzyme-linked immunosorbent assay to detect Type VII collagen autoantibodies in epidermolysis bullosa acquisita is useful not only for the diagnosis but also in the evaluation of disease severity.19,20
Anti-laminin 332 pemphigoid is a rare variant of mucous membrane pemphigoid characterized by circulating anti-laminin 332autoantibodies. It is associated with an increased risk of solid cancers. Anti-laminin 332 pemphigoid most frequently affects the oral cavity, conjunctiva, nasal cavity, ano-genital area, pharynx, larynx and esophagus.21-24
The commercially available dermatology BIOCHIP mosaic used in the present study includes a readily available salt-split skin which enables classification into floor and roof pattern staining. However, the use of salt-split skin cannot differentiate between the different pemphigoid diseases with floor pattern staining.
Alternatively, a panel of skin samples deficient for the particular basement membrane zone proteins, such as Type VII collagen or laminin 332, can be used to identify the target antigens. Availability of suitable skin that is deficient in basement membrane zone components is the limiting factor.25 Fluorescence overlay antigen mapping using laser scanning confocal microscopy is another method of differentiating pemphigoid disease. This method is of importance in patients in whom circulating autoantibodies are not detectable.26
Goyal et al. conducted a study with Indian patients and found that out of ten patients with floor pattern staining, six were epidermolysis bullosa acquisita and three were anti-p200 pemphigoid.15 A recent study conducted by Lau et al. showed that 115 (78.7%) of 141 patients with floor-staining pattern showed reactivity against the p200 antigen, followed by epidermolysis bullosa acquisita in 13 (9.2%) and anti-laminin 332 mucous membrane pemphigoid in four (2.8%) cases.27
In our study, all five patients with floor pattern staining on salt split skin showed positive reactivity to immunoblotting with the p200 antigen of dermal extracts as well as with the recombinant C-terminus of laminin ϒ1. Anti-p200 pemphigoid was first described in 1996 and is a rare pemphigoid disease.6,28 The p200 antigen is an acidic non-collagenous N-linked glycoprotein that is localized within the lower lamina lucida outside of hemidesmosome.29-33 In addition, the C-terminus of laminin ϒ1 is recognized in about 90% of anti-p200 pemphigoid patients.7,10 Clinically, anti-p200 pemphigoid is characterized by tense clear or hemorrhagic blisters on erythematous or normal skin resembling bullous pemphigoid.34,35 Oral and genital mucous membranes can be involved.36,37 Lesions usually heal without scarring and milia formation is also rare. Histopathology of anti-p200 pemphigoid shows a subepidermal bulla containing eosinophils, neutrophils38 and direct immunofluorescence microscopy of perilesional skin shows linear IgG and C3 staining similar to pemphigoid diseases. The lesional histopathologies of five anti-p200 pemphigoid patients were reviewed; four patients had predominant neutrophilic infiltrate in addition to subepidermal splitting while one patient did not have any predominance of inflammatory cells. Indirect immunofluorescence by BIOCHIP™ mosaic can be used to diagnose pemphigus vulgaris, pemphigus foliaceous and bullous pemphigoid and identify blister floor binding pemphigoid diseases. An extended BIOCHIP™ mosaic now allows the detection of antibodies against Type VII collagen and laminin 332,39-41 while a BIOCHIP™ for anti-200 pemphigoid is still lacking. It is important to diagnose anti-p200 pemphigoid because it has a benign course and can be treated with potent topical steroids, alone or in combination with dapsone.
Limitations
We could not perform serration pattern analysis of direct immunofluorescence in our patients.
Conclusion
Histopathology and direct immunofluorescence microscopy alone cannot differentiate between various entities of autoimmune subepidermal bullous diseases. A multistep approach using indirect immunofluorescence microscopy on salt-split skin followed by immunoblotting with dermal extract and recombinant laminin ϒ1 should be included in the diagnostic work-up to identify the target antigens in pemphigoid group of diseases.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- The diagnosis and treatment of autoimmune blistering skin diseases. Deutsch Ärztebl Int. 2011;108:399.
- [CrossRef] [PubMed] [Google Scholar]
- Reactivity of autoantibodies from patients with defined subepidermal bullous diseases against 1 mol/L salt-split skin: Specificity, sensitivity, and practical considerations. J Am Acad Dermatol. 1996;35:398-403.
- [CrossRef] [Google Scholar]
- Antiepiligrin cicatricial pemphigoid: A subepithelial bullous disorder. Arch Dermatol. 1994;130:1521-9.
- [CrossRef] [PubMed] [Google Scholar]
- Antiepiligrin cicatricial pemphigoid: The first case report from Japan. J Am Acad Dermatol. 1996;34:940-2.
- [CrossRef] [Google Scholar]
- A novel subepidermal blistering disease with autoantibodies to a 200-kDa antigen of the basement membrane zone. J Investig Dermatol. 1996;106:465-70.
- [CrossRef] [PubMed] [Google Scholar]
- Anti-laminin gamma-1 pemphigoid. Proc Natl Acad Sci. 2009;106:2800-5.
- [CrossRef] [PubMed] [Google Scholar]
- Serological diagnosis of autoimmune bullous skin diseases: Prospective comparison of the BIOCHIP mosaic-based indirect immunofluorescence technique with the conventional multi-step single test strategy. Orphanet J Rare Dis. 2012;7:49.
- [CrossRef] [PubMed] [Google Scholar]
- Development of a simple enzyme-linked immunosorbent assay for the detection of autoantibodies in anti-p200 pemphigoid. Br J Dermatol. 2011;164:76-82.
- [CrossRef] [PubMed] [Google Scholar]
- Pathogenicity of autoantibodies in anti-p200 pemphigoid. PLoS One. 2012;7:e41769.
- [CrossRef] [PubMed] [Google Scholar]
- Serration pattern analysis for differentiating epidermolysis bullosa acquisita from other pemphigoid diseases. J Am Acad Dermatol. 2018;78:754-9.
- [CrossRef] [PubMed] [Google Scholar]
- The n-vs-u-serration is a learnable criterion to differentiate pemphigoid from epidermolysis bullosa acquisita in direct immunofluorescence serration pattern analysis. Br J Dermatol. 2013;169:100-5.
- [CrossRef] [PubMed] [Google Scholar]
- Identification of the skin basement-membrane autoantigen in epidermolysis bullosa acquisita. N Engl J Med. 1984;310:1007-13.
- [CrossRef] [PubMed] [Google Scholar]
- Epidermolysis bullosa acquisita and anti-p200 pemphigoid as major subepidermal autoimmune bullous diseases diagnosed by floor binding on indirect immunofluorescence microscopy using human salt-split skin. Indian J Dermatol Venereol Leprol. 2017;83:550.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical features and diagnosis of epidermolysis bullosa acquisita. Expert Rev Clin Immunol. 2017;13:157-69.
- [CrossRef] [PubMed] [Google Scholar]
- The many faces of epidermolysis bullosa acquisita after serration pattern analysis by direct immunofluorescence microscopy. Br J Dermatol. 2011;165:92-8.
- [CrossRef] [PubMed] [Google Scholar]
- International bullous diseases group: consensus on diagnostic criteria for epidermolysis bullosa acquisita. Br J Dermatol. 2018;179:30-41.
- [CrossRef] [PubMed] [Google Scholar]
- Serum levels of anti-type VII collagen antibodies detected by enzyme-linked immunosorbent assay in patients with epidermolysis bullosa acquisita are correlated with the severity of skin lesions. J Eur Acad Dermatol Venereol. 2013;27:e224-30.
- [CrossRef] [PubMed] [Google Scholar]
- Development of an ELISA for rapid detection of anti-type VII collagen autoantibodies in epidermolysis bullosa acquisita. J Investig Dermatol. 1997;108:68-72.
- [CrossRef] [PubMed] [Google Scholar]
- Anti-epiligrin cicatricial pemphigoid and relative risk for cancer. Lancet. 2019;357:1850-1.
- [CrossRef] [Google Scholar]
- A case of anti-epiligrin cicatricial pemphigoid associated with lung carcinoma and severe laryngeal stenosis: Review of Japanese cases and evaluation of risk for internal malignancy. J Dermatol. 2004;31:10-5.
- [CrossRef] [PubMed] [Google Scholar]
- A sensitive and specific assay for the serological diagnosis of antilaminin 332 mucous membrane pemphigoid. Br J Dermatol. 2019;180:149-56.
- [CrossRef] [PubMed] [Google Scholar]
- Immunofluorescence serration pattern analysis as a diagnostic criterion in antilaminin-332 mucous membrane pemphigoid: Immunopathological findings and clinical experience in 10 Dutch patients. Br J Dermatol. 2011;165:815-22.
- [CrossRef] [PubMed] [Google Scholar]
- Antigen identification using skin deficient in basement-membrane protein: A novel tool for the diagnosis of subepidermal immunobullous diseases. Clin Exp Dermatol. 2013;38:289-94.
- [CrossRef] [PubMed] [Google Scholar]
- Bullous pemphigoid and epidermolysis bullosa acquisita: Differentiation by fluorescence overlay antigen mapping. Arch Dermatol. 1996;132:151-7.
- [CrossRef] [PubMed] [Google Scholar]
- Anti-p200 pemphigoid is the most common pemphigoid disease with serum antibodies against the dermal side by indirect immunofluorescence microscopy on human salt-split skin. J Am Acad Dermatol. 2019;81:1195-7.
- [CrossRef] [PubMed] [Google Scholar]
- From anti-p200 pemphigoid to anti-laminin γ1 pemphigoid. J Dermatol. 2010;37:231-8.
- [CrossRef] [PubMed] [Google Scholar]
- Immunoglobulin G deposition to nonhemidesmosomal lamina lucida and early neutrophil involvement are characteristic features in a case of anti-p200 pemphigoid. Br J Dermatol. 2013;168:647-55.
- [CrossRef] [PubMed] [Google Scholar]
- Coexistence of psoriasis and an unusual IgG-mediated subepidermal bullous dermatosis: Identification of a novel 200-kDa lower lamina lucida target antigen. Br J Dermatol. 1996;134:340-6.
- [CrossRef] [PubMed] [Google Scholar]
- Autoantibodies in anti-p200 pemphigoid stain skin lacking laminin 5 and type VII collagen. Br J Dermatol. 2000;143:1043-9.
- [CrossRef] [PubMed] [Google Scholar]
- Anti-p200 pemphigoid: Diagnosis and treatment of a case presenting as an inflammatory subepidermal blistering disease. J Am Acad Dermatol. 2002;46:786-9.
- [CrossRef] [PubMed] [Google Scholar]
- The autoantigen of anti-p200 pemphigoid is an acidic noncollagenous N-linked glycoprotein of the cutaneous basement membrane. J Investig Dermatol. 2003;121:1402-8.
- [CrossRef] [PubMed] [Google Scholar]
- A case of epidermolysis bullosa acquisita with autoantibody to anti-p200 pemphigoid antigen and exfoliative esophagitis. Dermatology. 2006;212:381-4.
- [CrossRef] [PubMed] [Google Scholar]
- Relapse-associated autoantibodies to BP180 in a patient with anti-p200 pemphigoid. Clin Exp Dermatol. 2010;35:614-7.
- [CrossRef] [PubMed] [Google Scholar]
- A severe and refractory case of anti-p200 pemphigoid resulting in multiple skin ulcers and scar formation. Dermatology. 2009;218:265-71.
- [CrossRef] [PubMed] [Google Scholar]
- A case of anti-p200 pemphigoid: evidence for a different pathway in neutrophil recruitment compared with bullous pemphigoid. Br J Dermatol. 2009;160:462-4.
- [CrossRef] [PubMed] [Google Scholar]
- Histopathology of anti-p200 pemphigoid. Am J Dermatopathol. 2007;29:119-24.
- [CrossRef] [PubMed] [Google Scholar]
- A sensitive and specific assay for the serological diagnosis of antilaminin 332 mucous membrane pemphigoid. Br J Dermatol. 2019;180:149-56.
- [CrossRef] [PubMed] [Google Scholar]
- Sensitive and specific assays for routine serological diagnosis of epidermolysis bullosa acquisita. J Am Acad Dermatol. 2013;68:e89-95.
- [CrossRef] [PubMed] [Google Scholar]
- Multicenter prospective study on multivariant diagnostics of autoimmune bullous dermatoses using the BIOCHIPTM technology. J Am Acad Dermatol. 2020;83:1315-22.
- [CrossRef] [PubMed] [Google Scholar]






