Translate this page into:
Antihypertensives in dermatology Part II - Cutaneous adverse reactions to antihypertensives
Correspondence Address:
P. S. S. Ranugha
Department of Dermatology and Venereology, JSS Medical College and Hospital, JSS University, MG Road, Mysore - 570 004, Karnataka
India
| How to cite this article: Ranugha P, Betkerur JB. Antihypertensives in dermatology Part II - Cutaneous adverse reactions to antihypertensives. Indian J Dermatol Venereol Leprol 2018;84:137-147 |
Abstract
Antihypertensive drugs are prescribed frequently and can cause cutaneous adverse reactions. The exact incidence and frequency of these reactions are unknown. Multiple antihypertensive drug consumption has contributed to a substantial increase in the number of cutaneous adverse reactions to them. Thus, there is a need for dermatologists and physicians to be aware of the wide range of available antihypertensives and the type of reactions that can be expected. This review article focuses on the various clinical presentations that have been implicated or associated with them. The diagnosis and management have been discussed in brief.Introduction
Antihypertensives are used extensively for hypertension as well as other indications including migraine, alopecia, hemangioma, etc., Cutaneous adverse drug reactions to them are common, but the exact incidence and frequency are unknown. Turk et al. found these drugs to be the incriminating cause in 8.5% of hospitalized patients, preceded by antibiotics, nonsteroidal anti-inflammatory drugs (NSAIDs) and anticonvulsants.[1] Upadhayai et al. found that 2% of such patients developed drug reactions.[2] The information obtained from the Danish National Board of Health's Committee on Adverse Drug Reactions showed that 10–60% of reactions caused by antihypertensives are dermatological.[3] There is lack of comprehensive data on the incidence and different types of cutaneous reactions occurring with common and newer antihypertensives. Relevant articles from the PubMed database were collected and analyzed. Case control studies or meta-analyses which showed significant association of drugs with any cutaneous adverse drug reaction were highlighted. Observations have been mentioned as reports.
Some classes of antihypertensives are commonly associated with certain reactions.
- Angiotensin converting enzyme (ACE) inhibitors: The overall incidence of adverse effects is estimated at 28%, approximately half of which are cutaneous. The common cutaneous reactions are potentially life threatening angioedema, pruritus, bullous eruptions, urticaria, photosensitivity and hair loss.[4]
- Calcium channel blockers: The most common reactions are gingival hyperplasia (21%) and flushing (10%). Other reactions described are facial or truncal telangiectasia, photosensitivity, new-onset psoriasis (as well as exacerbation), purpuric exanthems, pemphigoid, subacute cutaneous lupus erythematosus, gynecomastia, erythromelalgia and oral ulcers. The frequency of these reactions may be as high as 48%. The more serious reactions associated are toxic epidermal necrolysis with diltiazem. Stevens–Johnson syndrome, erythema multiforme and exfoliative dermatitis have been associated with all three drugs in this class.[5] Reactions occur more frequently with diltiazem than others.[6]
- Beta blockers: The pathogenetic mechanism responsible is still obscure. It may be due to blockade of the epidermal cell and T-lymphocyte beta-receptors, rather than direct immunologic, allergic or toxic mechanisms.[7] Beta blockers have been commonly associated with lichenoid drug eruptions, eczematous and psoriasiform eruptions.[8]
- Diuretics: Thiazides can cause vasculitis, phototoxic/allergic reaction, erythema multiforme and eczema.[3] Furosemide can cause bullous pemphigoid as well as pseudoporphyria.
There are very few studies on the prevalence of cutaneous adverse drug reactions due to antihypertensives. An Indian study showed beta-blockers as the most common agent, followed by calcium channel blockers. The most common patterns observed were urticaria, followed by lichenoid drug eruptions.[2] In a Danish study, amiloride and hydrochlorthiazide had the highest number of cutaneous reactions.[3] The common and rare cutaneous adverse drug reactions reported with antihypertensives are tabulated [Table - 1]. The individual cutaneous adverse drug reaction patterns are discussed below.
Acute Generalized exanthematous pustulosis
Acute generalized exanthematous pustulosis is characterized by the rapid development of non-follicular, sterile pustules on an erythematous base. It is attributed to drugs in most cases. Systemic involvement with hepatic, renal or pulmonary insufficiency occurs in approximately 20% of the cases.[9] The eruption occurs 2 to 5 days after drug intake. Although antibiotics are the most common cause, a few cases with diltiazem [10] and terazosin hydrochloride [11] have been described. In a multinational case control study (EUROSCAR), which assessed the risk factors, diltiazem was found to be associated with a higher risk along with antibiotics.[12] T-cell involvement is suggested by positive patch test reactions to the suspected drug.[13] They may directly orchestrate a neutrophilic inflammation by releasing the neutrophil attracting chemokine CXCL8.[14] Discontinuance of the drug is the only treatment necessary, although corticosteroids may be needed in some cases.
Angioedema
ACE inhibitors are the leading cause of drug-induced angioedema, with an incidence of 0.1–0.2%. This is non-immunological and occurs in predisposed individuals. It is caused by accumulation of vasoactive mediators like bradykinin due to reduced activity of angiotensin-converting enzyme.[3] It is never accompanied by urticaria, can start years after beginning the treatment, and can recur irregularly while under treatment.[15] It has varying clinical presentations including isolated involvement of lip or penis,[16] one side of the tongue,[17] or small bowel involvement.[18] Common agents which have been implicated are enalapril,[3] lisinopril [2],[3] and alacepril.[19] They may also cause increased frequency, intensity and duration of bouts of idiopathic angioedema during long-term use.[20] Icatibant, a bradykinin receptor antagonist has been shown to accelerate the resolution of ACE inhibitor induced angioedema.[21] Renin inhibitor aliskerin and angiotensin receptor blockers (losartan, valsartan, candesartan) have lower risk of causing angioedema. It is less severe and occurs earlier compared to ACE inhibitors.[22],[23] There is less than a 10% chance for these groups of drugs to cause angioedema compared to patients who had angioedema due to ACE inhibitors.[23] Angioedema has also been described in children, most commonly to the dihydropyridine group of calcium channel blockers (amlodipine and nicardipine).[24]
Annular Erythema
Hydrochlorthiazide and spironolactone have caused erythema annulare centrifugum like eruptions.[25],[26]
Bullous Eruptions
Pseudoporphyria
Pseudoporphyria is a porphyria like blistering on exposed areas in the absence of abnormal porphyrin metabolism. It may be caused by high dose furosemide,[27] torsemide,[28] bumetanide,[29] flutamide,[30] chlorthalidone [31] and dyazide (combination of triamterene and hydrochlorthiazide).[32]
Pemphigus group (pemphigus foliaceus and pemphigus vulgaris)
Drug-related pemphigus can be of two types, (i) induced pemphigus, in which exogenous factors play a major role and (ii) triggered pemphigus, in which endogenous factors play a major role. Induced pemphigus is usually caused by thiol group of drugs such as captopril. It has a long incubation period of up to one year and mostly resembles pemphigus foliaceus or pemphigus vegetans. Triggered pemphigus mimics pemphigus vulgaris, has a shorter incubation period (128 days average) and is usually caused by non-thiol drugs.[33] The various non-thiol antihypertensives which trigger this are mentioned in [Table - 2][34],[35],[36],[37],[38]. Thiol drugs provoke acantholysis in vitro possibly by increasing the activity of plasminogen activators.[39] An active amide group in the molecule of non-thiol drugs may be responsible for inducing pemphigus.[40]

The diagnosis of drug-induced pemphigus is challenging. It resembles idiopathic pemphigus in clinical findings, histopathology and immunofluorescence, thus making it difficult to differentiate the two.[34] Approximately 70–90% of patients have a positive direct immunofluorescence.[41] More than half of the cases caused by thiol drugs remit following drug withdrawal, whereas only 15% of those caused by non-thiol drugs do so.[33] The treatment starts with the immediate withdrawal of the suspected drug (s). Medium to high dose of systemic steroids (about 2/3 of the dose normally used in idiopathic pemphigus) is usually recommended until all symptoms of active disease disappear. In most cases, remission can be achieved within weeks, and steroid doses may be gradually tapered down to zero after a few months.[42]
Drug-induced bullous pemphigoid
Drugs may induce anti basement membrane zone antibody production by acting as haptens that bind to proteins in the lamina lucida and change their antigenic properties. They may stimulate an autoimmune response by structurally modifying molecules and uncovering hidden epitopes. In drug induced bullous pemphigoid, patients tend to be younger. The clinical presentation is heterogenous and variable. Nikolsky sign may be positive in some cases. Tissue bound and circulating anti basement membrane zone IgG antibodies may be absent. Additional antibodies such as intercellular or anti-epidermal cytoplasmic antibodies may be detected. Histopathologically, there may be perivascular infiltration of lymphocytes with a few eosinophils and neutrophils, intraepidermal vesicles with foci of necrotic keratinocytes and thrombi in dermal vessels.[43],[44] Marked eosinophilia may be found in serum as well as tissue. Apart from the classical presentation, milder forms are devoid of erythematous bases. Unusual presentations in the form of scarring plaques, nodules with bullae, or excoriations located on scalp and extremities (papular and nodular pemphigoid) have been described.[45] It can mimic other entities such as bullous erythema multiforme [46] and pemphigus (overlapping variants). Some cases are short-lived whereas others become chronic, in the form of drug-triggered bullous pemhigoid.[43]
Various antihypertensives can induce bullous pemphigoid [Table - 2].[47],[48] Drugs such as furosemide and enalapril are most likely to have an association with bullous pemphigoid, proven by rechallenge.[44] Bastuji-Garin et al. reported a strong association with neuroleptics and diuretics (mainly aldosterone antagonists).[48] ACE inhibitors, anticoagulants and diuretics were found to be commonly used by patients suffering from bullous pemphigoid.[49] In a recent case-control study that included 86 patients, loop diuretics were found to be used more frequently. This association was independent of age, cerebrovascular disease, dementia, hypertension or ischemic heart disease.[50] Mucous membrane pemphigoid has been observed with atenolol,[51] isolated ocular cicatricial pemphigoid with ophthalmic anti-glaucoma preparations,[52] and anogenital cicatricial pemphigoid with clonidine.[53] Lichen planus pemphigoides has been reported with captopril and ramipril. Its course tends to be much more indolent but it responds well to treatment.[54] Linear IgA bullous dermatosis has been induced by captopril.[55]
The possibility of a drug etiology must be considered in all patients suffering from bullous pemphigoid as most patients respond rapidly to treatment and do not experience relapses after the withdrawal of the suspect medication.[43]
Cutaneous Vasculitis
Approximately 20% cases of cutaneous small vessel vasculitis are an adverse reaction to drugs and most represent hypersensitivity vasculitis.[56] Therapeutic agents from virtually every pharmacologic class have been implicated. The offending drugs can be generally categorized into: Anti-neutrophil cytoplasmic antibody (ANCA) associated and ANCA negative group. Development of systemic vasculitis may take a few months to years following exposure. The ANCA negative group usually presents with cutaneous involvement within a few days to weeks after drug exposure.[57] An average lag period of 28.9 days was found in an Indian study.[2] Hydralazine has been incriminated in ANCA positive vasculitis, lupus erythematosus like syndrome and digital gangrene.[58] [Table - 2] depicts the various antihypertensives that cause cutaneous small vessel vasculitis.[58],[59],[60],[61],[62],[63],[64],[65],[66]
Blood eosinophilia is found in almost 80% patients with drug-induced systemic vasculitis. However, it is less than 25% in patients who have only cutaneous involvement. The presence of tissue eosinophilia on histology is suggestive of a drug induced vasculitis.[67] Apart from withdrawal of the suspected drug, oral steroids may be needed in cases with systemic involvement. We need to be aware of possible cross reactions (among diuretics, calcium channel blockers) while substituting a drug.
Drug Reaction With Eosinophilia and Systemic Symptoms
This is a potentially life threatening adverse drug reaction with an estimated mortality of 10%, most commonly from fulminant hepatitis with hepatic necrosis. It is seen in children and adults most often as a morbilliform cutaneous eruption with fever, lymphadenopathy, hematological and multiorgan abnormalities. It has a late onset and long duration compared to other drug reactions, with a latent period of 2–6 weeks.[68] There may be associated vesicles, bullae, atypical targetoid plaques and purpura. Sterile follicular and non-follicular pustules may be evident.[69] The rash may progress to involve nearly the entire surface of the skin, producing an exfoliative dermatitis or erythroderma. This can be associated with mucosal involvement such as cheilitis, erosions, erythematous pharynx and enlarged tonsils.[70]
Even though anticonvulsants, sulphonamides and allopurinol are common causes, ACE inhibitors (captopril, enalapril, ramipril [71]), beta-blockers (atenolol, celiprolol) and spironolactone [72] are reported to induce drug reaction with eosinophilia and systemic symptoms. Prolonged systemic corticosteroid therapy may be required. A gradual taper of this therapy over 3–6 months after clinical and laboratory stabilization is recommended to avoid relapse.[73] Incomplete recurrences with structurally unrelated culprit drugs are a frequent phenomenon in such patients.[74]
Erythema Multiforme
Less than 10% of cases of erythema multiforme are drug induced.[75] Although NSAIDs, sulphonamides and antibiotics are common culprits, isolated reports have been described with furosemide, indapamide, carvedilol, metoprolol, fenoterol, nifedepine, amlodipine, diltiazem, cardiazem, topical dorzolamide and candesartan axetil.[76],[77],[78],[79],[80],[81],[82],[83],[84],[85] It may be associated with a flu-like prodrome. Blisters and mucosal involvement is more prominent than herpes simplex virus associated erythema multiforme. The course is self limiting with no recurrences after stopping the drug. A mortality rate of 5–15% has been reported in severe cases.[75]
Exanthematous Eruptions
Exanthematous eruptions with various morphological and localization patterns are the most frequently encountered cutaneous adverse drug reactions. They can occur after almost any drug, usually within 2-3 weeks of drug administration. They may be accompanied by fever, pruritus and eosinophilia.[86] The course of these benign exanthems lasts for a few days to some weeks. If the drug is continued, an exfoliative dermatitis may develop. Occasionally, the eruption subsides despite continuation of the medication.[86] Immunological effector mechanisms include drug-specific CD4+ T cells, various chemokines and cytokines.[87] Exanthematous drug eruption has been reported with diltiazem and valsartan.[88],[89] Telmisartan has caused symmetrical drug related intertriginous and flexural exanthem.[90]
Eczematous Eruptions
Eczematous drug reactions may be localized or generalized. The term 'endogenous contact eczema' refers to the occurrence of an eczematous contact drug reaction following primary sensitization by oral therapy. These may develop following therapy with methyldopa and clonidine.[91] Among ACE inhibitors, captopril has been shown to cause an eczematous reaction, confirmed in many cases with patch testing, without any cross-reactivity with enalapril , lisinopril or benazepril.[92] The latency period can vary from a few months to several years. A lag period of 4–30 months was observed in a study.[93]
Eyelid dermatitis was seen with the use of beta-blocker eyedrops (timolol, befunolol, carteolol, propranolol, practalol) with cross-sensitivity among these. The proposed hypothesis of cross-sensitivity is primary metabolism of the drug to a common aldehyde.[94] Stasis dermatitis has been described with amlodipine.[95] Topical diltiazem used for anal fissures is known to cause contact dermatitis.[96] In a study of 23 cases of localized and generalized eczematous drug reactions caused by antihypertensives, the class of drugs implicated were ACE inhibitors, angiotensin receptor blockers and hydrochlorthiazide in combination with ACE inhibitors or angiotensin receptor blockers.[93] Extensive allergic contact dermatitis has been seen in factory workers coming in contact with alprenolol.[97] Localized contact allergy with transdermal clonidine has also been described.[98]
Eczematoid Photosensitive Reactions
Most systemic drug photosensitivity is due to phototoxic mechanisms. Different patterns of phototoxic reactions occur in the skin, including an immediate prickling/burning sensation, urticaria, sunburn-like reaction, late onset erythema, dermatitis, skin fragility and telangiectasias.[99] Drucker and Rosen, suggested ten drugs to be considered potent photosensitisers, of which hydrochlorthiazide was the only antihypertensive.[100] Other drugs include diuretics (triamterene, furosemide), ACE inhibitors (ramipril, enalapril, quinapril), calcium channel blockers (nifedepine), beta-blockers (tilisolol), angiotensin receptor blockers (valsartan), centrally acting agents (clonidine, methyldopa), valsartan and methyl-dopa have been described to cause photosensitivity in the past, but these are mostly individual case reports. Amlodipine and nifedepine can cause photodistributed facial telangiectasia.[99],[100] In a study of 62 cases of thiazide induced photosensitivity, eczematous presentation was found to be the most common.[101] In most cases, phototesting revealed an abnormal response to UVA rays alone, or to both UVA and UVB. For systemic drug phototoxicity, the key investigation is phototesting with a monochromator and drug rechallenge phototesting. Photopatch testing is needed in suspected cases of photo-allergy. Drug-induced photosensitivity is usually managed by stopping the suspected drug. Other measures are sometimes necessary, including phototherapy using wavelengths that do not elicit the response.[99]
Erythroderma
Exfoliative dermatitis is one of the most dangerous cutaneous adverse drug reactions. Captopril,[102] lisinopril,[103] diltiazem,[104] amlodipine, timolol eye drops [105] and glyceryl trinitrate [106] have caused erythroderma. Interstitial granulomatous drug reaction secondary to enalapril presenting as erythroderma has been reported.[107] The latency period is highly variable, ranging from a few days to several months.
Erythromelalgia
This reaction has been related to nifedipine, diltiazem, verapamil and nicardipine. It is characterized by intermittent, usually symmetrical burning pain, warmth and dermal erythema of the extremities. The symptoms are ameliorated by cooling the extremities.[5] The time lapse between the first dose of the drug and its occurrence varied from eight weeks to a year. The time from discontinuation of the drug to resolution ranged from one to fourteen days.[108]
Fixed Drug Eruptions
A fixed drug eruption characteristically recurs at the same site every time the drug is administered. The number of sites affected may increase with each exposure. Although this is rare following antihypertensives, diltiazem, enalapril and amlodipine have been implicated.[109] Isolated reports of fixed drug eruption secondary to propranolol,[110] atenolol,[111] bisoprolol, nifedepine,[112] hydralazine [113] and indapamide [114] have been described. The latency period was 2 months to 19.6 months in an Indian study.[2]
Hyperpigmentation
Diltiazem has been implicated as the cause of photodistributed hyperpigmentation in several reports. The interval from initiation of diltiazem to the onset varies from a few months to years. Histologically, the changes are consistent with a lichenoid dermatitis that show basal vacuolar alteration and prominent pigment incontinence.[100],[108],[115] Kubo et al. propose that diltiazem associated photodistributed hyperpigmentation must be a specific type of drug-induced photosensitive lichenoid eruption, probably in the UVB range.[116] Photoprotection, hydroquinone and tacrolimus cream have been tried. Pigmentation of skin predominantly over sun-exposed areas and pigmentation of oral mucosa have been described after one year of amlodipine intake.[117]
Lichenoid Drug Eruptions
Lichenoid drug eruptions tend to be extensive and may develop weeks or months after initiation of therapy [Figure - 1]. Lesions may be more psoriasiform than those seen in classic lichen planus. Oral involvement is rare. There may be atypical features such as marked scaling, eczematization, hypertrophic lesions and a tendency to more intense residual hyperpigmentation.[118] The antihypertensives which may cause lichenoid drug eruption are enumerated in [Table - 2].[119],[120],[121],[122],[123],[124] Cross-reactivity among beta-blockers has not been demonstrated.[121] Valsartan caused linear lichenoid eruption [125], whereas lichenoid nail dystrophy was reported to angiotensin receptor blockers in another case.[126] Bullous lesions were seen with labetalol, and penile involvement with propranolol.[121] Photolichenoid eruption has been reported with hydrochlorthiazide, enalapril [119] and inhaled tiotropium bromide.[127] Isolated oral eruptions have been seen with calcium channel blockers, ACE inhibitors and beta-blockers.[128] Oral ulcerative lichen planus was observed with methyldopa.[129] The intra-oral sites of predilection include the posterior buccal mucosa, tongue, floor of mouth, palate and alveolar ridges. There appears to be a preference for unilateral distribution. They are nearly identical to oral lichen planus clinically, histologically and immunologically.[130] McCartan and McCreary have provided a structured system for reporting oral lichenoid drug eruption cases.[131]
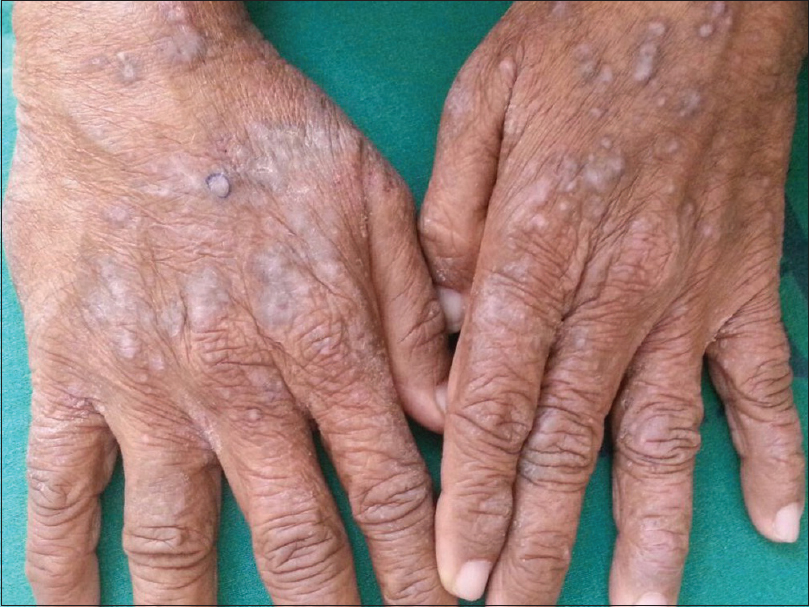 |
| Figure 1: Lichenoid papules and plaques over the dorsal aspect of both hands |
The lag period is variable and the latency period ranges from one month to two years (19.6 months average). Resolution of the skin and mucosal eruptions may be slow and variable, with a resolution time of 1–4 months.[2] Withdrawal of the drug and symptomatic treatment is often sufficient. Severe cases may require corticosteroid therapy as in idiopathic lichen planus.
Lupus Erythematosus
Drug-induced lupus erythematosus is defined as a lupus erythematosus like syndrome temporally related to continuous drug exposure, which resolves after discontinuation of the offending drug. Similar to idiopathic lupus erythematosus, this can be divided into systemic lupus erythematosus (SLE), subacute cutaneous lupus erythematosus and chronic cutaneous lupus erythematosus.[132] It is believed that Fas-dependent apoptosis of epidermal basal keratinocytes plays an important role. A reduction of immunohistochemical expression of Bcl-2, an antiapoptotic protein, has been demonstrated in lesional skin along the epidermal basal layer among such patients.[133] In general, old patients are affected and there is no sex predilection as seen in idiopathic SLE. The time between drug exposure to onset of symptoms varies from a month to more than a decade.[134]
Skin involvement is less frequent in drug-induced SLE, although its exact incidence remains controversial. Certain non-specific cutaneous manifestations such as purpura, erythema nodosum and photosensitivity are frequently present in drug-induced SLE than its idiopathic counterpart. Features such as malar rash, discoid lesions, mucosal ulcers, alopecia and Raynaud's phenomenon are usually absent in drug-induced SLE. Other non-specific features such as urticaria, urticarial vasculitis and signs of necrotising vasculitis may be considered characteristic of drug-induced lupus erythematosus.[132],[134],[135] Fever, arthralgia, myalgia, pleurisy and pericarditis are present, whereas renal and central nervous system involvement is rare. Anti-nuclear antibody and anti-histone antibodies are positive, whereas Anti-ds DNA is usually negative and complement levels are normal. Deposition of immunoreactants in uninvolved skin is rare. A negative ANA test should not automatically preclude a diagnosis of drug-induced lupus erythematosus, particularly if the patient has other autoantibodies associated with SLE or drug-induced lupus erythematosus.[132],[134] Hydralazine induced lupus erythematosus with Sweet's syndrome has been reported.[136]
Of the antihypertensives implicated in drug-induced SLE [Table - 2][134],[135], hydralazine and methyldopa have a definite association while others have a probable or possible association.[137] A matched, nested, case-control study conducted in the United Kingdom to investigate drugs causing lupus erythematosus found a causal relationship only for carbamazepine, minocycline and possibly hydralazine.[138] Resolution or marked improvement of the symptoms generally occurs within 2–5 weeks of drug withdrawal, although some patients may require NSAIDS or low dose systemic steroids. Immunosuppressive drugs may be needed in severe cases with renal or neurological involvement. Patients who develop ANA positivity during treatment need not have the drug stopped. They do not require treatment unless they have clinical features of lupus erythematosus.[139] Drug-induced subacute lupus erythematosus [Table - 2][132],[134] is similar to its idiopathic counterpart, both clinically and serologically.[134],[140] In most cases, there is spontaneous resolution within weeks of drug withdrawal. The Anti Ro/SS-A antibodies may remain positive even after resolution of disease activity.[140]
Pityriasis Rosea-Like Eruptions
An Italian series reported cases of pityriasis rosea linked to ACE inhibitors, alone or in combination with hydrochlorothiazide. They had also reported a case of pityriasis rosea with hydrochlorothiazide plus losartan.[141]
Palmoplantar Keratoderma
Losartan has been shown to cause palmoplantar hyperkeratosis, which resolved after withdrawal of the drug.[142]
Purpura
Hydralazine [143] can cause pigmented purpuric dermatosis. A case of amlodipine induced Schamberg's purpura occurred eight years after starting treatment, and resolved within three months of stopping the drug.[2] Chlorthiazide and hydrochlorthiazide have been shown to cause thrombocytopenia and purpura.[144] Frictional purpuric eruption may occur with angiotensin receptor blockers.[145]
Psoriasiform Eruptions
The antihypertensives that are strongly related to psoriasis are beta blockers and ACE inhibitors. Other drugs also have been reported to induce or aggravate psoriasis, but the evidence is less strong. In general, most drugs tend to exacerbate psoriasis rather than induce it [146] Drug-induced psoriasiform eruption tends to occur de novo in patients with no prior personal or family history of psoriasis.[147] The eruptions appear 1–18 months after initiation of the drugs.[148] However, a lag period of two years has been observed.[2] Psoriasiform eruptions clear after several weeks of drug withdrawal,[148] but drug aggravated psoriasis may not clear completely. Drug-induced psoriasiform eruption is not true psoriasis. The lesions are less red, less thick and less scaly [Figure - 2]. The knees and elbows tend to be spared. Histopathologically, they lack neutrophils or Munro's microabscesses. Both cardioselective and non-cardioselective beta blockers can aggravate psoriasis or induce a psoriasiform rash.[149] Topical application of timolol in the treatment of open angle glaucoma has been reported to induce psoriasis and transform psoriasis vulgaris into psoriatic erythroderma, by systemic absorption via the conjunctiva.[150]
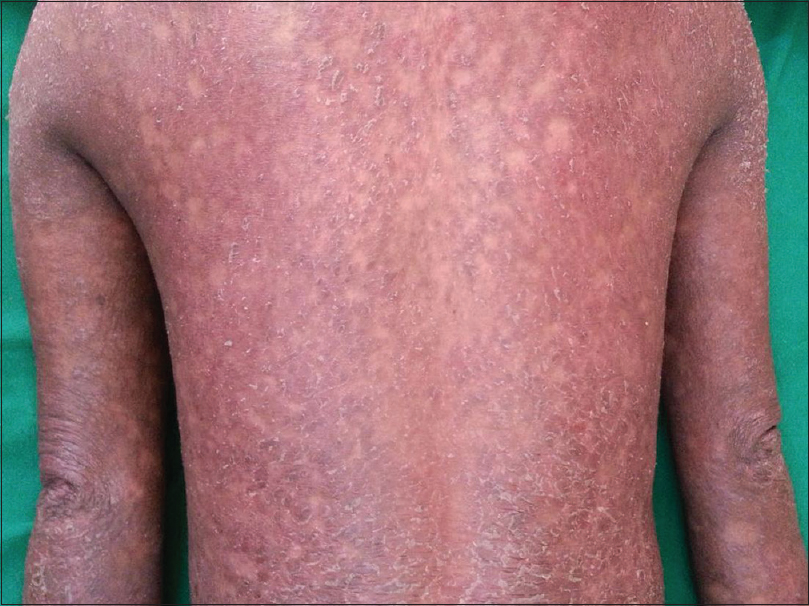 |
| Figure 2: Psoriasiform drug eruption with diffuse erythema and scaling over the back and upper limbs |
Blockade of beta 2 receptors leads to a decrease of cAMP, causing a decrease in intracellular calcium, excessive release of enzymes by lymphocytes, neutrophils and macrophages. This consequently increases cellular proliferation and lack of differentiation.[151] ACE inhibitors have been implicated in case-control and case-crossover studies.[146],[151] They act by altering the kinin-kallikrein arachidonic acid system, which may lead to increased concentrations of inflammatory metabolites, thus inducing psoriasis. Other drugs with a weak association include angiotensin receptor blockers,[152] calcium channel blockers,[153] clonidine [154] and urapidil (α1 adrenergic blocker).[155]
A prospective cohort study on the risk of psoriasis taking individual antihypertensives found that only beta blockers were associated with an increased risk after regular use for six or more years.[156] On the other hand, in a population based case-control study, no increased or altered risk of psoriasis was found with beta blockers or other antihypertensives.[157] Propranolol,[158] atenolol,[159] pindolol,[3] ramipril [160] and candesartan [161] have been shown to induce generalized pustular psoriasis. Captopril, enalapril and perindopril have caused palmoplantar psoriasis and palmoplantar pustulosis.[162] Oxprenolol has been shown to exacerbate psoriatic arthropathy.[163] Diltiazem has also precipitated psoriatic erythroderma.[164]
Pseudolymphomatous Drug Eruptions
Cutaneous pseudolymphomas can be either of T-cell or B-cell origin on histology. Characteristically, anticonvulsant induced pseudolymphoma hypersensitivity syndromes develop soon after the drug has been started, usually within two to eight weeks.[165] However, cases have developed as late as seven years.[166] There are numerous reports of antihypertensive induced pseudolymphomatous drug eruptions in the literature [Table - 2].[167],[168],[169],[170],[171],[172],[173],[174],[175] They resolve in 1–32 weeks of discontinuing the medication.[175] It is postulated that the drug may promote an aberrant immune response to an antigen, which may be the drug itself, or some other stimulus. Failure of lesions to resolve months after drug discontinuation should raise suspicion of a malignant process. Appropriate investigations must be done, as true lymphomas may occasionally develop.
Toxic Epidermal Necrolysis
Although antihypertensive associated toxic epidermal necrolysis is extremely rare, isolated reports secondary to sodium nitroprusside,[176] amlodipine,[177] captopril,[178] carvedilol,[179] oral minoxidil,[180] indapamide,[181] alfuzosin [182] and hydralazine [183] have been described. Timolol, dalfuzomide, and latanoprost eye drops [184] may also induce this condition. A multinational case-control study conducted in Europe found that beta blockers, ACE inhibitors, calcium channel blockers, thiazide diuretics and furosemide were not associated with a detectable risk of Stevens Johnson syndrome or toxic epidermal necrolysis.[185] A similar result was found for thiazides [186] and ACE inhibitors.[187]
Hair and Nail Changes
Propranolol,[188] metoprolol [189] and certain ophthalmic beta blockers [190] can cause alopecia. Diazoxide and minoxidil can cause hypertrichosis.[191] Drug-induced changes in hair colour, usually occurs 3–12 months after the onset of treatment,[192] and has been described with verapamil.[193] Onycholysis may occur with captopril, thiazides, proctalol and indapamide.[194]
Oral Changes
Dry mouth has been reported in approximately 20% of hypertensives treated with beta-adrenergic blockers. They may decrease the total protein content of saliva. The administration of ACE inhibitors may cause dry mouth due to reduction of the salivary flow rate. Diuretics may cause dry mouth by dehydration and salivary gland hypofunction. Alpha 1 adrenergic agents may result in altered saliva composition and secretion rates. Dry mouth is reversible on drug discontinuation.
ACE inhibitors are associated with taste disturbances. Impaired or salty taste is a frequent complaint with captopril. These tend to be self limiting and reversible within two to three months even if the drug is continued.[195] Malic acid 1% spray improved antihypertensive induced xerostomia and stimulated the production of saliva.[196] Buccal ulceration and aphthous-like ulcers have been reported with beta blockers, ACE inhibitors (captopril, enalapril), angiotensin receptor blockers (losartan), nicorandil and methyldopa.[195],[197],[198] Nicorandil can cause oral, anal and mucocutaneous ulcerations. It may rarely cause leg ulceration without mucosal involvement.[199] Within the calcium channel blockers family, nifedipine, diltiazem, verapamil and amlodipine can cause gingival hyperplasia. Tissue enlargement typically occurs within one to three months of therapy, usually beginning in the interdental papillae. Its pathogenesis is traced back to the increased production of collagen by gingival fibroblasts, which may account for the lack of rapid resolution after drug discontinuation.[200]
Diagnosis of Cutaneous Adverse Drug Reactions to Antihypertensive Drugs
Numerous methods for causality assessment in adverse drug reactions have been published. They fall into three broad categories – expert judgement, algorithms and probabilistic methods. Due to problems of reproducibility and validity, no single method is universally accepted.[201] At present there are no specific tests that can predict the capacity of drugs to induce allergic reactions, or of the susceptibility of individuals to experience an allergic reaction. Skin testing, especially patch test, was found to be a useful screening method if the reaction was exanthema. It was also useful if antimicrobial, cardiovascular or antiepileptic drugs were suspected.[202] Oral rechallenge needs to be considered when patch tests are negative, but cannot be performed in case of severe drug reactions. As the latency of the reaction is prolonged and variable with many antihypertensives, the utility of an oral rechallenge in such situations is doubtful. In-vitro cytokine release tests like interferon gamma release test and the cell scan apparatus to detect activation of lymphocytes may have a role in diagnosing cutaneous drug eruptions in the future.[203]
Conclusion
Cutaneous adverse drug reactions to antihypertensives are common. The time of onset and presentation is highly variable. Hypertensive patients are receiving multiple drug therapy nowadays, more than what used to be the norm a decade ago. The ever increasing list of newer antihypertensives has contributed to a substantial increase in the number of adverse reactions, especially cutaneous. Hence, dermatologists need to be aware of the various antihypertensives and the cutaneous adverse drug reactions that can occur due to them. Large scale population based prospective studies might give us further insights into the frequency, as well as the clinical presentations that can be expected. Further studies are necessary on tests for causality assessment of such reactions.
Financial support and sponsorship
Nil. No financial support or sponsorship has been received.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Turk BG, Gunaydin A, Ertam I, Ozturk G. Adverse cutaneous drug reactions among hospitalized patients: Five year surveillance. Cutan Ocul Toxicol 2013;32:41-5.
[Google Scholar]
|
| 2. |
Upadhayai JB, Nangia AK, Mukhija RD, Misra M, Mohan L, Singh KK. Cutaneous reactions due to antihypertensive drugs. Indian J Dermatol 2006;51:189-91.
[Google Scholar]
|
| 3. |
Thestrup-Pedersen K. Adverse reactions in the skin from anti-hypertensive drugs. Dan Med Bull 1987;34 Suppl 1:3-5.
[Google Scholar]
|
| 4. |
Steckelings UM, Artuc M, Wollschläger T, Wiehstutz S, Henz BM. Angiotensin-converting enzyme inhibitors as inducers of adverse cutaneous reactions. Acta Derm Venereol 2001;81:321-5.
[Google Scholar]
|
| 5. |
Ioulios P, Charalampos M, Efrossini T. The spectrum of cutaneous reactions associated with calcium antagonists: A review of the literature and the possible etiopathogenic mechanisms. Dermatol Online J 2003;9:6.
[Google Scholar]
|
| 6. |
Tuchinda P, Kulthanan K, Khankham S, Jongjarearnprasert K, Dhana N. Cutaneous adverse reactions to calcium channel blockers. Asian Pac J Allergy Immunol 2014;32:246-50.
[Google Scholar]
|
| 7. |
Faure M, Hermier C, Perrot H. Cutaneous reactions to propranolol (author's transl). Ann Dermatol Venereol 1979;106:161-5.
[Google Scholar]
|
| 8. |
Bonnetblanc JM. Drug eruptions caused by beta-blockers. Ann Med Interne (Paris) 1984;135:639-41.
[Google Scholar]
|
| 9. |
Szatkowski J, Schwartz RA. Acute generalized exanthematous pustulosis (AGEP): A review and update. J Am Acad Dermatol 2015;73:843-8.
[Google Scholar]
|
| 10. |
Serrão V, Caldas Lopes L, Campos Lopes JM, Lobo L, Ferreira A. Acute generalized exanthematous pustulosis associated with diltiazem. Acta Med Port 2008;21:99-102.
[Google Scholar]
|
| 11. |
Speck LM, Wilkerson MG, Perri AJ, Kelly BC. Acute generalized exanthematous pustulosis caused by terazosin hydrochloride. J Drugs Dermatol 2008;7:395-7.
[Google Scholar]
|
| 12. |
Gensch K, Hodzic-Avdagic N, Megahed M, Ruzicka T, Kuhn A. Acute generalized exanthematous pustulosis with confirmed type IV allergy. Report of 3 cases. Hautarzt 2007;58:250-2, 254-5.
[Google Scholar]
|
| 13. |
Britschgi M, Pichler WJ. Acute generalized exanthematous pustulosis, a clue to neutrophil-mediated inflammatory processes orchestrated by T cells. Curr Opin Allergy Clin Immunol 2002;2:325-31.
[Google Scholar]
|
| 14. |
Sidoroff A, Dunant A, Viboud C, Halevy S, Bavinck JN, Naldi L, et al. Risk factors for acute generalized exanthematous pustulosis (AGEP)-results of a multinational case-control study (EuroSCAR). Br J Dermatol 2007;157:989-96.
[Google Scholar]
|
| 15. |
Inomata N. Recent advances in drug-induced angioedema. Allergol Int 2012;61:545-57.
[Google Scholar]
|
| 16. |
Miller DG, Sweis RT, Toerne TS. Penile angioedema developing after 3 years of ACEI therapy. J Emerg Med 2012;43:273-5.
[Google Scholar]
|
| 17. |
Leung E, Hanna MY, Tehami N, Francombe J. Isolated unilateral tongue oedema: the adverse effect of angiotensin converting enzyme inhibitors. Curr Drug Saf 2012;7:382-3.
[Google Scholar]
|
| 18. |
Smet BS, De Kock I, De Backer AI, Verstraete K. Angioedema of the small bowel caused by angiotensin converting enzyme inhibitor. JBR BTR 2013;96:41.
[Google Scholar]
|
| 19. |
Katoh H, Itagaki T, Suzuki K, Obata Y, Adachi Y, Doi M, et al. Successful extubation in a patient with alacepril-induced tongue angioedema. Masui 2010;59:519-22.
[Google Scholar]
|
| 20. |
Kozel MM, Mekkes JR, Bos JD. Increased frequency and severity of angio-oedema related to long-term therapy with angiotensin-converting enzyme inhibitor in two patients. Clin Exp Dermatol 1995;20:60-1.
[Google Scholar]
|
| 21. |
Gallitelli M, Alzetta M. Icatibant: a novel approach to the treatment of angioedema related to the use of angiotensin-converting enzyme inhibitors. Am J Emerg Med 2012;30:1664.e1-2.
[Google Scholar]
|
| 22. |
Toh S, Reichman ME, Houstoun M, Ross Southworth M, Ding X, Hernandez AF, et al. Comparative risk for angioedema associated with the use of drugs that target the renin-angiotensin-aldosterone system. Arch Intern Med 2012;172:1582-9.
[Google Scholar]
|
| 23. |
Beavers CJ, Dunn SP, Macaulay TE. The role of angiotensin receptor blockers in patients with angiotensin-converting enzyme inhibitor-induced angioedema. Ann Pharmacother 2011;45:520-4.
[Google Scholar]
|
| 24. |
Pierce WA, Hederman AD, Gordon CJ, Ostrenga AR, Herrington B. Angioedema associated with dihydropyridine calcium-channel blockers in a child with Burkitt lymphoma. Am J Health Syst Pharm 2011;68:402-6.
[Google Scholar]
|
| 25. |
Goette DK, Beatrice E. Erythema annulare centrifugum caused by hydrochlorothiazide-induced interstitial nephritis. Int J Dermatol 1988;27:129-30.
[Google Scholar]
|
| 26. |
Carsuzaa F, Pierre C, Dubegny M. Erythema annulare centrifugum caused by aldactone. Ann Dermatol Venereol 1987;114:375-6.
[Google Scholar]
|
| 27. |
Breier F, Feldmann R, Pelzl M, Gschnait F. Pseudoporphyria cutanea tarda induced by furosemide in a patient undergoing peritoneal dialysis. Dermatology 1998;197:271-3.
[Google Scholar]
|
| 28. |
Pérez-Bustillo A, Sánchez-Sambucety P, Suárez-Amor O, Rodríiguez-Prieto MA. Torsemide-induced pseudoporphyria. Arch Dermatol 2008;144:812-3.
[Google Scholar]
|
| 29. |
Leitao EA, Person JR. Bumetanide-induced pseudoporphyria. J Am Acad Dermatol 1990;23:129-30.
[Google Scholar]
|
| 30. |
Schmutz JL, Barbaud A, Tréchot P. Flutamide and pseudoporphyria. Ann Dermatol Venereol 1999;126:374.
[Google Scholar]
|
| 31. |
Baker EJ, Reed KD, Dixon SL. Chlorthalidone-induced pseudoporphyria: clinical and microscopic findings of a case. J Am Acad Dermatol 1989;21 (5 Pt 1):1026-9.
[Google Scholar]
|
| 32. |
Motley RJ. Pseudoporphyria due to dyazide in a patient with vitiligo. BMJ 1990;300:1468.
[Google Scholar]
|
| 33. |
Brenner S, Bialy-Golan A, Ruocco V. Drug-induced pemphigus. Clin Dermatol 1998;16:393-7.
[Google Scholar]
|
| 34. |
Ong CS, Cook N, Lee S. Drug-related pemphigus and angiotensin converting enzyme inhibitors. Australas J Dermatol 2000;41:242-6.
[Google Scholar]
|
| 35. |
Patterson CR, Davies MG. Pemphigus foliaceus: an adverse reaction to lisinopril. J Dermatolog Treat 2004;15:60-2.
[Google Scholar]
|
| 36. |
Godard W, Lambert D, Gavanou J, Chapuis JL. Pemphigus induced by treatment with a propanolol-meprobamate combination. Ann Dermatol Venereol 1980;107:1213-6.
[Google Scholar]
|
| 37. |
Bae YI, Yun SJ, Lee SC, Park GT, Lee JB. Pemphigus foliaceus induced by an angiotensin II receptor blocker. Clin Exp Dermatol 2008;33:721-3.
[Google Scholar]
|
| 38. |
Bayramgürler D, Erçin C, Apaydin R, Unal G. Indapamide-induced pemphigus foliaceus. J Dermatolog Treat 2001;12:175-7.
[Google Scholar]
|
| 39. |
Lombardi ML, de Angelis E, Rossano F, Ruocco V. Imbalance between plasminogen activator and its inhibitors in thiol-induced acantholysis. Dermatology 1993;186:118-22.
[Google Scholar]
|
| 40. |
Wolf R, Brenner S. An active amide group in the molecule of drugs that induce pemphigus: a casual or causal relationship? Dermatology 1994;189:1-4.
[Google Scholar]
|
| 41. |
Wolf R, Tamir A, Brenner S. Drug-induced versus drug-triggered pemphigus. Dermatologica 1991;182:207-10.
[Google Scholar]
|
| 42. |
Ruocco V, Sacerdoti G. Pemphigus and bullous pemphigoid due to drugs. Int J Dermatol 1991;30:307-12.
[Google Scholar]
|
| 43. |
Lo Schiavo A, Ruocco E, Brancaccio G, Caccavale S, Ruocco V, Wolf R. Bullous pemphigoid: etiology, pathogenesis, and inducing factors: facts and controversies. Clin Dermatol 2013;31:391-9.
[Google Scholar]
|
| 44. |
Liu HN, Su WP, Rogers RS 3rd. Clinical variants of pemphigoid. Int J Dermatol 1986;25:17-27.
[Google Scholar]
|
| 45. |
Park KY, Kim BJ, Kim MN. Amlodipine-associated bullous pemphigoid with erythema multiforme-like clinical features. Int J Dermatol 2011;50:637-9.
[Google Scholar]
|
| 46. |
Stavropoulos PG, Soura E, Antoniou C. Drug-induced pemphigoid: a review of the literature. J Eur Acad Dermatol Venereol 2014;28:1133-40.
[Google Scholar]
|
| 47. |
Femiano F. Mucocutaneous bullous pemphigoid induced by valsartan. A clinical case. Minerva Stomatol 2003;52:187-90.
[Google Scholar]
|
| 48. |
Bastuji-Garin S, Joly P, Picard-Dahan C, Bernard P, Vaillant L, Pauwels C, et al. Drugs associated with bullous pemphigoid. A case-control study. Arch Dermatol 1996;132:272-6.
[Google Scholar]
|
| 49. |
Patsatsi A, Vyzantiadis TA, Chrysomallis F, Devliotou-Panagiotidou D, Sotiriadis D. Medication history of a series of patients with bullous pemphigoid from Northern Greece-observations and discussion. Int J Dermatol 2009;48:132-5.
[Google Scholar]
|
| 50. |
Lloyd-Lavery A, Chi CC, Wojnarowska F, Taghipour K. The associations between bullous pemphigoid and drug use: a UK case-control study. JAMA Dermatol 2013;149:58-62.
[Google Scholar]
|
| 51. |
Kanjanabuch P, Arporniem S, Thamrat S, Thumasombut P. Mucous membrane pemphigoid in a patient with hypertension treated with atenolol: a case report. J Med Case Rep 2012;6:373.52. Kubo M, Sakuraba T, Arai Y, Nakazawa M. A case of suspected drug-induced ocular pemphigoid. Nippon Ganka Gakkai Zasshi 2001;105:189-92.
[Google Scholar]
|
| 52. |
van Joost T, Faber WR, Manuel HR. Drug-induced anogenital cicatricial pemphigoid. Br J Dermatol 1980;102:715-8.
[Google Scholar]
|
| 53. |
Harting MS, Hsu S. Lichen planus pemphigoides: a case report and review of the literature. Dermatol Online J 2006;12:10.
[Google Scholar]
|
| 54. |
Friedman IS, Rudikoff D, Phelps RG, Sapadin AN. Captopril-triggered linear IgA bullous dermatosis. Int J Dermatol 1998;37:608-12.
[Google Scholar]
|
| 55. |
Chen KR, Carlson JA. Clinical approach to cutaneous vasculitis. Am J Clin Dermatol 2008;9:71-92.
[Google Scholar]
|
| 56. |
ten Holder SM, Joy MS, Falk RJ. Cutaneous and systemic manifestations of drug-induced vasculitis. Ann Pharmacother 2002;36:130-47.
[Google Scholar]
|
| 57. |
Yokogawa N, Vivino FB. Hydralazine-induced autoimmune disease: comparison to idiopathic lupus and ANCA-positive vasculitis. Mod Rheumatol 2009;19:338-47.
[Google Scholar]
|
| 58. |
Pulido-Pérez A, Avilés-Izquierdo JA, Suárez-Fernández R. Cutaneous vasculitis. Actas Dermosifiliogr 2012;103:179-91.
[Google Scholar]
|
| 59. |
Pavlovic MD, Dragojevic Simic V, Zolotarevski L, Zecevic RD, Vesic S. Cutaneous vasculitis induced by carvedilol. J Eur Acad Dermatol Venereol 2007;21:1004-5.
[Google Scholar]
|
| 60. |
Miralles R, Pedro-Botet J, Farré M, Rubiés-Prat J. Captopril and vasculitis. Ann Intern Med 1988;109:514.
[Google Scholar]
|
| 61. |
Carrington PR, Sanusi ID, Zahradka S, Winder PR. Enalapril-associated erythema and vasculitis. Cutis 1993;51:121-3.
[Google Scholar]
|
| 62. |
Gupta S, Gandhi NM, Ferguson J. Cutaneous vasculitis secondary to ramipril. J Drugs Dermatol 2004;3:81-2.
[Google Scholar]
|
| 63. |
Meissner M, Kaufmann R. Annular leukocytoclastic vasculitis after the administration of an amlodipine generic. J Eur Acad Dermatol Venereol 2009;23:238-9.
[Google Scholar]
|
| 64. |
Kuo M, Winiarski N, Garella S. Nonthrombocytopenic purpura associated sequentially with nifedipine and diltiazem. Ann Pharmacother 1992;26:1089-90.
[Google Scholar]
|
| 65. |
Piérard Franchimont C, Henry F, Piérard GE. Severe pustular and polymorphous vasculitis caused by losartan. Ann Dermatol Venereol 2001;128(10 Pt 1):1040-2.
[Google Scholar]
|
| 66. |
Bahrami S, Malone JC, Webb KG, Callen JP. Tissue eosinophilia as an indicator of drug-induced cutaneous small-vessel vasculitis. Arch Dermatol 2006;142:155-61.
[Google Scholar]
|
| 67. |
Husain Z, Reddy BY, Schwartz RA. DRESS syndrome: Part I. Clinical perspectives. J Am Acad Dermatol 2013;68:693.e1-14.
[Google Scholar]
|
| 68. |
Bocquet H, Bagot M, Roujeau JC. Drug-induced pseudolymphoma and drug hypersensitivity syndrome (Drug Rash with Eosinophilia and Systemic Symptoms: DRESS). Semin Cutan Med Surg 1996;15:250-7.
[Google Scholar]
|
| 69. |
Piñana E, Lei SH, Merino R, Melgosa M, De La Vega R, Gonzales-Obeso E, et al. DRESS-syndrome on sulfasalazine and naproxen treatment for juvenile idiopathic arthritis and reactivation of human herpevirus 6 in an 11-year-old Caucasian boy. J Clin Pharm Ther 2010;35:365-70.
[Google Scholar]
|
| 70. |
Pileri A, Brunasso AM, Tilz H, Wolf P, Massone C. Ramipril-induced drug reaction with eosinophilia and systemic symptoms (DRESS). Eur J Dermatol 2011;21:624-5.
[Google Scholar]
|
| 71. |
Ghislain PD, Bodarwe AD, Vanderdonckt O, Tennstedt D, Marot L, Lachapelle JM. Drug-induced eosinophilia and multisystemic failure with positive patch-test reaction to spironolactone: DRESS syndrome. Acta Derm Venereol 2004;84:65-8.
[Google Scholar]
|
| 72. |
Spriet S, Banks TA. Drug reaction with eosinophilia and systemic symptoms syndrome. Allergy Asthma Proc 2015;36:501-5.
[Google Scholar]
|
| 73. |
Picard D, Vellar M, Janela B, Roussel A, Joly P, Musette P. Recurrence of drug-induced reactions in DRESS patients. J Eur Acad Dermatol Venereol 2015;29:801-4.
[Google Scholar]
|
| 74. |
Samim F, Auluck A, Zed C, Williams PM. Erythema multiforme: a review of epidemiology, pathogenesis, clinical features, and treatment. Dent Clin North Am 2013;57:583-96.
[Google Scholar]
|
| 75. |
Zugerman C, La Voo EJ. Erythema multiforme caused by oral furosemide. Arch Dermatol 1980;116:518-9.
[Google Scholar]
|
| 76. |
Gales BJ, Gales MA. Erythema multiforme and angioedema with indapamide and sertraline. Am J Hosp Pharm 1994;51:118-9.
[Google Scholar]
|
| 77. |
Hong JA, Bisognano JD. Metoprolol succinate therapy associated with erythema multiforme. Cardiol J 2009;16:82-3.
[Google Scholar]
|
| 78. |
Sachs B, Renn C, al Masaoudi T, Merk HF. Fenoterol-induced erythema exudativum multiforme-like exanthem: demonstration of drug-specific lymphocyte reactivity in vivo and in vitro. Acta Derm Venereol 2001;81:368-9.
[Google Scholar]
|
| 79. |
Springuel P. Erythema multiforme and nifedipine. CMAJ 1997;156:90-1.
[Google Scholar]
|
| 80. |
Bewley AP, Feher MD, Staughton RC. Erythema multiforme following substitution of amlopidine for nifedipine. BMJ 1993;307:241.
[Google Scholar]
|
| 81. |
Berbis P, Alfonso MJ, Levy JL, Privat Y. Diltiazem associated erythema multiforme. Dermatologica 1989;179:90.
[Google Scholar]
|
| 82. |
Brown FH, Houston GD, Phillips M, Westbrook SD. Erythema multiforme following cardizem therapy: report of a case. Ann Dent 1989;48:39-40, 52.
[Google Scholar]
|
| 83. |
Munshi V, Ahluwalia H. Erythema multiforme after use of topical dorzolamide. J Ocul Pharmacol Ther 2008;24:91-3.
[Google Scholar]
|
| 84. |
Ejaz AA, Walsh JS, Wasiluk A. Erythema multiforme associated with candesartan cilexetil. South Med J 2004;97:614-5.
[Google Scholar]
|
| 85. |
Yawalkar N. Drug-induced exanthems. Toxicology 2005;209:131-4.
[Google Scholar]
|
| 86. |
Bircher AJ. Uncomplicated drug-induced disseminated exanthemas. Chem Immunol Allergy 2012;97:79-97.
[Google Scholar]
|
| 87. |
Ozturk G, Turk BG, Senturk B, Turkmen M, Kandiloglu G. Exanthematous drug eruption due to valsartan. Cutan Ocul Toxicol 2012;31:335-7.
[Google Scholar]
|
| 88. |
Cholez C, Trechot P, Schmutz JL, Faure G, Bene MC, Barbaud A. Maculopapular rash induced by diltiazem: allergological investigations in four patients and cross reactions between calcium channel blockers. Allergy 2003;58:1207-9.
[Google Scholar]
|
| 89. |
Ferreira O, Mota A, Morais P, Cunha AP, Azevedo F. Symmetrical drug-related intertriginous and flexural exanthema (SDRIFE) induced by telmisartan-hydrochlorothiazide. Cutan Ocul Toxicol 2010;29:293-5.
[Google Scholar]
|
| 90. |
Pirilä V. Endogenic contact eczema. Allerg Asthma (Leipz) 1970;16:15-9.
[Google Scholar]
|
| 91. |
Lluch-Bernal M, Novalbos A, Umpierrez A, Figueredo E, Bombin C, Sastre J. Cutaneous reaction to captopril with positive patch test and lack of cross-sensitivity to enalapril and benazepril. Contact Dermatitis 1998;39:316-7.
[Google Scholar]
|
| 92. |
Vena GA, Cassano N, Coco V, De Simone C. Eczematous reactions due to angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers. Immunopharmacol Immunotoxicol 2013;35:447-50.
[Google Scholar]
|
| 93. |
Giordano-Labadie F, Lepoittevin JP, Calix I, Bazex J. Contact allergy to beta blockaders in eye drops: cross allergy? Ann Dermatol Venereol 1997;124:322-4.
[Google Scholar]
|
| 94. |
Gosnell AL, Nedorost ST. Stasis dermatitis as a complication of amlodipine therapy. J Drugs Dermatol 2009;8:135-7.
[Google Scholar]
|
| 95. |
Leinonen PT, Riekki R, Oikarinen A. Contact allergy to diltiazem cream. Contact Dermatitis 2010;63:228-30.
[Google Scholar]
|
| 96. |
Ekenvall L, Forsbeck M. Contact eczema produced by a beta-adrenergic blocking agent (alprenolol). Contact Dermatitis 1978;4:190-4.
[Google Scholar]
|
| 97. |
Polster AM, Warner MR, Camisa C. Allergic contact dermatitis from transdermal clonidine in a patient with mycosis fungoides. Cutis 1999;63:154-5.
[Google Scholar]
|
| 98. |
Dawe RS, Ibbotson SH. Drug-induced photosensitivity. Dermatol Clin 2014;32:363-8, ix.
[Google Scholar]
|
| 99. |
Drucker AM, Rosen CF. Drug-induced photosensitivity: culprit drugs, management and prevention. Drug Saf 2011;34:821-37.
[Google Scholar]
|
| 100. |
Gómez-Bernal S, Alvarez-Pérez A, Rodríguez-Pazos L, Gutiérrez-González E, Rodríguez-Granados MT, Toribio J. Photosensitivity due to thiazides. Actas Dermosifiliogr 2014;105:359-66.
[Google Scholar]
|
| 101. |
O'Neill PG, Rajan N, Charlat ML, Bolli R. Captopril-related exfoliative dermatitis. Tex Med 1989;85:40-1.
[Google Scholar]
|
| 102. |
Schmutz JL, Barbaud A, Tréchot P. Lisinopril-induced erythroderma. Ann Dermatol Venereol 2009;136:486.
[Google Scholar]
|
| 103. |
Odeh M. Exfoliative dermatitis associated with diltiazem. J Toxicol Clin Toxicol 1997;35:101-4.
[Google Scholar]
|
| 104. |
Shelley WB, Shelley ED. Chronic erythroderma induced by beta-blocker (timolol maleate) eyedrops. J Am Acad Dermatol 1997;37(5 Pt 1):799-800.
[Google Scholar]
|
| 105. |
Ryan FP. Erythroderma due to peritrate and glyceryl trinitrate. Br J Dermatol 1972;87:498-500.
[Google Scholar]
|
| 106. |
Chen YC, Hsiao CH, Tsai TF. Interstitial granulomatous drug reaction presenting as erythroderma: remission after discontinuation of enalapril maleate. Br J Dermatol 2008;158:1143-5.
[Google Scholar]
|
| 107. |
Drenth JP, Michiels JJ, Van Joost T, Vuzevski VDVerapamil-induced secondary erythermalgiaBr J Dermatol, 127(3):292-4, 1992.
[Google Scholar]
|
| 108. |
Savin JA. Current causes of fixed drug eruption in the UK. Br J Dermatol 2001;145:667-8.
[Google Scholar]
|
| 109. |
Zaccaria E, Gualco F, Drago F, Rebora A. Fixed drug eruption due to propranolol. Acta Derm Venereol 2006;86:371.
[Google Scholar]
|
| 110. |
Belhadjali H, Trimech O, Youssef M, Elhani I, Zili J. Fixed drug eruption induced by atenolol. Clin Cosmet Investig Dermatol 2009;1:37-9.
[Google Scholar]
|
| 111. |
Alcalay J, David M, Sandbank M. Cutaneous reactions to nifedipine. Dermatologica 1987;175:191-3.
[Google Scholar]
|
| 112. |
Sehgal VN, Gangwani OP. Hydralazine-induced fixed drug eruption. Int J Dermatol 1986;25:394.
[Google Scholar]
|
| 113. |
De Barrio M, Tornero P, Zubeldia JM, Sierra Z, Matheu V, Herrero T. Fixed drug eruption induced by indapamide. Cross-reactivity with sulfonamides. J Investig Allergol Clin Immunol 1998;8:253-5.
[Google Scholar]
|
| 114. |
Scherschun L, Lee MW, Lim HW. Diltiazem-associated photodistributed hyperpigmentation: a review of 4 cases. Arch Dermatol 2001;137:179-82.
[Google Scholar]
|
| 115. |
Kubo Y, Fukumoto D, Ishigami T, Hida Y, Arase S. Diltiazem-associated photodistributed hyperpigmentation: report of two Japanese cases and published work review. J Dermatol 2010;37:807-11.
[Google Scholar]
|
| 116. |
Erbagci Z. Amlodipine associated hyperpigmentation. Saudi Med J 2004;25:103-5.
[Google Scholar]
|
| 117. |
Almeyda J, Levantine A. Drug reactions. XVI. Lichenoid drug eruptions. Br J Dermatol 1971;85:604-7.
[Google Scholar]
|
| 118. |
Sehgal VN, Srivastava G, Sharma S, Sehgal S, Verma P. Lichenoid tissue reaction/interface dermatitis: recognition, classification, etiology, and clinicopathological overtones. Indian J Dermatol Venereol Leprol 2011;77:418-29.
[Google Scholar]
|
| 119. |
Wasada T, Nanko H, Iwasaki N, Iwamoto Y. Severe protracted lichenoid eruption and hyperuricemia following administration of alacepril. Intern Med 1999;38:164.
[Google Scholar]
|
| 120. |
Fessa C, Lim P, Kossard S, Richards S, Peñas PF. Lichen planus-like drug eruptions due to ß-blockers: a case report and literature review. Am J Clin Dermatol 2012;13:417-21.
[Google Scholar]
|
| 121. |
Menter MA. Hypertrichosis lanuginosa and a lichenoid eruption due to diazoxide therapy. Proc R Soc Med 1973;66:326-7.
[Google Scholar]
|
| 122. |
Majmudar V, Al-Dulaimi H, Dodd H. Lichenoid drug eruption secondary to treatment with nicorandil? Clin Exp Dermatol 2008;33:193-4.
[Google Scholar]
|
| 123. |
Koh MJ, Seah PP, Tay YK, Mancer K. Lichenoid drug eruption to terazosin. Br J Dermatol 2008;158:426-7.
[Google Scholar]
|
| 124. |
Gencoglan G, Ceylan C, Kazandi AC. Linear lichenoid drug eruption induced by valsartan. Clin Exp Dermatol 2009;34:e334-5.
[Google Scholar]
|
| 125. |
Bories A, Denis P. Lichenoid nail dystrophy induced by angiotensin 2 receptor antagonists. Ann Dermatol Venereol 2005;132:265-7.
[Google Scholar]
|
| 126. |
Pérez-Pérez L, Cabanillas M, Pereiro Ferreirós MM, Peteiro C, Toribio J. Photosensitive lichenoid eruption and inhaled tiotropium bromide. Dermatology 2007;214:97-8.
[Google Scholar]
|
| 127. |
Nagaraj E, Eswar P, Kaur RP. Etiogenic study on oral lichenoid reactions among Tamil Nadu population: a prospective cohort study. Indian J Dent Res 2013;24:309-15.
[Google Scholar]
|
| 128. |
España A, Torrelo A, Soria C, Ledo A. Erosive lichen of the oral mucosa caused by alphamethyldopa and hydrochlorothiazide. Med Clin (Barc) 1990;94:559.
[Google Scholar]
|
| 129. |
Woo V, Bonks J, Borukhova L, Zegarelli D. Oral lichenoid drug eruption: a report of a pediatric case and review of the literature. Pediatr Dermatol 2009;26:458-64.
[Google Scholar]
|
| 130. |
McCartan BE, McCreary CE. Oral lichenoid drug eruptions. Oral Dis 1997;3:58-63.
[Google Scholar]
|
| 131. |
Marzano AV, Vezzoli P, Crosti C. Drug-induced lupus: an update on its dermatologic aspects. Lupus 2009;18:935-40.
[Google Scholar]
|
| 132. |
Baima B, Sticherling M. Apoptosis in different cutaneous manifestations of lupus erythematosus. Br J Dermatol 2001;144:958-66.
[Google Scholar]
|
| 133. |
Vasoo S. Drug-induced lupus: an update. Lupus 2006;15:757-61.
[Google Scholar]
|
| 134. |
Vedove CD, Del Giglio M, Schena D, Girolomoni G. Drug-induced lupus erythematosus. Arch Dermatol Res 2009;301:99-105.
[Google Scholar]
|
| 135. |
Cartee TV, Chen SC. Sweet syndrome associated with hydralazine-induced lupus erythematosus. Cutis 2012;89:121-4.
[Google Scholar]
|
| 136. |
Sarzi-Puttini P, Atzeni F, Capsoni F, Lubrano E, Doria A. Drug-induced lupus erythematosus. Autoimmunity 2005;38:507-18.
[Google Scholar]
|
| 137. |
Schoonen WM, Thomas SL, Somers EC, Smeeth L, Kim J, Evans S, et al. Do selected drugs increase the risk of lupus? A matched case-control study. Br J Clin Pharmacol 2010;70:588-96.
[Google Scholar]
|
| 138. |
Aguirre Zamorano MA, López Pedrera R, Cuadrado Lozano MJ. Drug-induced lupus. Med Clin (Barc) 2010;135:124-9.
[Google Scholar]
|
| 139. |
Lowe GC, Henderson CL, Grau RH, Hansen CB, Sontheimer RD. A systematic review of drug-induced subacute cutaneous lupus erythematosus. Br J Dermatol 2011;164:465-72.
[Google Scholar]
|
| 140. |
Atzori L, Pinna AL, Ferreli C, Aste N. Pityriasis rosea-like adverse reaction: review of the literature and experience of an Italian drug-surveillance center. Dermatol Online J 2006;12:1.
[Google Scholar]
|
| 141. |
Calvo M, Fernández-Guarino M, Martín-Saez E, Carrillo R, Garate M. Palmoplantar hyperkeratosis associated with losartan. Actas Dermosifiliogr 2006;97:463-6.
[Google Scholar]
|
| 142. |
Sardana K, Sarkar R, Sehgal VN. Pigmented purpuric dermatoses: an overview. Int J Dermatol 2004;43:482-8.
[Google Scholar]
|
| 143. |
Ball P. Thrombocytopenia and purpura in patients receiving chlorothiazide and hydrochlorothiazide. J Am Med Assoc 1960;173:663-5.
[Google Scholar]
|
| 144. |
Foti C, Carbonara AM, Guida S, Antelmi A, Mazzocca A, Romita P, et al. Frictional purpuric eruption associated with angiotensin II receptor blockers. Dermatol Ther 2014;27:97-100.
[Google Scholar]
|
| 145. |
Fry L, Baker BS. Triggering psoriasis: the role of infections and medications. Clin Dermatol 2007;25:606-15.
[Google Scholar]
|
| 146. |
Kim GK, Del Rosso JQ. Drug-provoked psoriasis: is it drug induced or drug aggravated? understanding pathophysiology and clinical relevance. J Clin Aesthet Dermatol 2010;3:32-8.
[Google Scholar]
|
| 147. |
Cohen AD, Bonneh DY, Reuveni H, Vardy DA, Naggan L, Halevy S. Drug exposure and psoriasis vulgaris: case-control and case-crossover studies. Acta Derm Venereol 2005;85:299-303.
[Google Scholar]
|
| 148. |
Heng MC, Heng MK. Beta-adrenoceptor antagonist-induced psoriasiform eruption. Clinical and pathogenetic aspects. Int J Dermatol 1988;27:619-27.
[Google Scholar]
|
| 149. |
Puig L, Goñi FJ, Roqué AM, Bordas FD, de Moragas JM. Psoriasis induced by ophthalmic timolol preparations. Am J Ophthalmol 1989;108:455-6.
[Google Scholar]
|
| 150. |
Ockenfels HM, Nussbaum G, Schultewolter T, Mertins K, Wagner SN, Goos M. Tyrosine phosphorylation in psoriatic T cells is modulated by drugs that induce or improve psoriasis. Dermatology 1995;191:217-25.
[Google Scholar]
|
| 151. |
Marquart-Elbaz C, Grosshans E, Lipsker D, Lipsker D. Sartans, angiotensin II receptor antagonists, can induce psoriasis. Br J Dermatol 2002;147:617-8.
[Google Scholar]
|
| 152. |
Kitamura K, Kanasashi M, Suga C, Saito S, Yoshida S, Ikezawa Z. Cutaneous reactions induced by calcium channel blocker: high frequency of psoriasiform eruptions. J Dermatol 1993;20:279-86.
[Google Scholar]
|
| 153. |
Wilkin J. Exacerbation of psoriasis during clonidine therapy. Arch Dermatol 1981;117:4.
[Google Scholar]
|
| 154. |
Takehara Y, Igawa K, Satoh T, Yokozeki H. Psoriasiform eruption induced by alpha1-adrenergic blocker, urapidil. J Eur Acad Dermatol Venereol 2007;21:577-8.
[Google Scholar]
|
| 155. |
Wu S, Han J, Li WQ, Qureshi AA. Hypertension, antihypertensive medication use, and risk of psoriasis. JAMA Dermatol 2014;150:957-63.
[Google Scholar]
|
| 156. |
Brauchli YB, Jick SS, Curtin F, Meier CR. Association between beta-blockers, other antihypertensive drugs and psoriasis: population-based case-control study. Br J Dermatol 2008;158:1299-307.
[Google Scholar]
|
| 157. |
Hu CH, Miller AC, Peppercorn R, Farber EM. Generalized pustular psoriasis provoked by propranolol. Arch Dermatol 1985;121:1326-7.
[Google Scholar]
|
| 158. |
Wakefield PE, Berger TG, James WD. Atenolol-induced pustular psoriasis. Arch Dermatol 1990;126:968-9.
[Google Scholar]
|
| 159. |
Thakor P, Padmanabhan M, Johnson A, Pararajasingam T, Thakor S, Jorgensen W. Ramipril-induced generalized pustular psoriasis: case report and literature review. Am J Ther 2010;17:92-5.
[Google Scholar]
|
| 160. |
Kawamura A, Ochiai T. Candesartan cilexetil induced pustular psoriasis. Eur J Dermatol 2003;13:406-7.
[Google Scholar]
|
| 161. |
Eriksen JG, Christiansen JJ, Asmussen I. Postulosis palmoplantaris caused by angiotensin-converting enzyme inhibitors. Ugeskr Laeger 1995;157:3335-6.
[Google Scholar]
|
| 162. |
Macfarlane DG, Settas L. Acute psoriatric arthropathy precipitated by oxprenolol. Ann Rheum Dis 1984;43:102-4.
[Google Scholar]
|
| 163. |
Lavrijsen AP, Van Dijke C, Vermeer BJ. Diltiazem-associated exfoliative dermatitis in a patient with psoriasis. Acta Derm Venereol 1986;66:536-8.
[Google Scholar]
|
| 164. |
Kardaun SH, Scheffer E, Vermeer BJ. Drug-induced pseudolymphomatous skin reactions. Br J Dermatol 1988;118:545-52.
[Google Scholar]
|
| 165. |
Wood GS, Tung RM, Haeffner AC, Crooks CF, Liao S, Orozco R, et al. Detection of clonal T-cell receptor gamma gene rearrangements in early mycosis fungoides/Sezary syndrome by polymerase chain reaction and denaturing gradient gel electrophoresis (PCR/DGGE). J Invest Dermatol 1994;103:34-41.
[Google Scholar]
|
| 166. |
Furness PN, Goodfield MJ, MacLennan KA, Stevens A, Millard LG. Severe cutaneous reactions to captopril and enalapril; histological study and comparison with early mycosis fungoides. J Clin Pathol 1986;39:902-7.
[Google Scholar]
|
| 167. |
Kabashima R, Orimo H, Hino R, Nakashima D, Kabashima K, Tokura Y. CD30-positive T-cell pseudolymphoma induced by amlodipine. J Eur Acad Dermatol Venereol 2008;22:1522-4.
[Google Scholar]
|
| 168. |
Ploysangam T, Breneman DL, Mutasim DF. Cutaneous pseudolymphomas. J Am Acad Dermatol 1998;38 (6 Pt 1):877-95.
[Google Scholar]
|
| 169. |
Henderson CA, Shamy HK. Atenolol-induced pseudolymphoma. Clin Exp Dermatol 1990;15:119-20.
[Google Scholar]
|
| 170. |
Viraben R, Lamant L, Brousset P. Losartan-associated atypical cutaneous lymphoid hyperplasia. Lancet 1997;350:1366.
[Google Scholar]
|
| 171. |
Sawada Y, Yoshiki R, Kawakami C, Fukamachi S, Sugita K, Nakamura M, et al. Valsartan-induced drug eruption followed by CD30+pseudolymphomatous eruption. Acta Derm Venereol 2010;90:521-2.
[Google Scholar]
|
| 172. |
Jahan-Tigh RR, Huen AO, Lee GL, Pozadzides JV, Liu P, Duvic M. Hydrochlorothiazide and cutaneous T cell lymphoma: prospective analysis and case series. Cancer 2013;119:825-31.
[Google Scholar]
|
| 173. |
Shelley WB, Shelley ED. Pseudolymphoma at site of clonidine patch. Lancet 1997;350:1223-4.
[Google Scholar]
|
| 174. |
Magro CM, Crowson AN. Drug-induced immune dysregulation as a cause of atypical cutaneous lymphoid infiltrates: a hypothesis. Hum Pathol 1996;27:125-32.
[Google Scholar]
|
| 175. |
Kilic M, Ozturk F, Genc G, Guner SN, Yildiz L, Sancak R. Sodium nitroprusside and toxic epidermal necrolysis. Asian Pac J Allergy Immunol 2012;30:243-5.
[Google Scholar]
|
| 176. |
Baetz BE, Patton ML, Guilday RE, Reigart CL, Ackerman BH. Amlodipine-induced toxic epidermal necrolysis. J Burn Care Res 2011;32:e158-60.
[Google Scholar]
|
| 177. |
Alkurtass DA, Al-Jazairi AS. Possible captopril-induced toxic epidermal necrolysis. Ann Pharmacother 2003;37:380-3.
[Google Scholar]
|
| 178. |
Vlahovic-Palcevski V, Milic S, Hauser G, Protic A, Zupan Z, Reljic M, et al. Toxic epidermal necrolysis associated with carvedilol treatment. Int J Clin Pharmacol Ther 2010;48:549-51.
[Google Scholar]
|
| 179. |
Karaoui LR, Chahine-Chakhtoura C. Fatal toxic epidermal necrolysis associated with minoxidil. Pharmacotherapy 2009;29:460-7.
[Google Scholar]
|
| 180. |
Partanen J, Pohjola-Sintonen S, Mäkijärvi M, Härmä M. Toxic epidermal necrolysis due to indapamide. Arch Dermatol 1993;129:793.
[Google Scholar]
|
| 181. |
Wang YS, Tay YK, Kwok C. Toxic epidermal necrolysis caused by alfuzosin, an alpha1-adrenoceptor antagonist. Arch Dermatol 2006;142:938.
[Google Scholar]
|
| 182. |
Chan JC, Yap DY, Yeung CK. Hydralazine-induced toxic epidermal necrolysis in a patient on continuous ambulatory peritoneal dialysis. J Clin Pharm Ther 2014;39:322-4.
[Google Scholar]
|
| 183. |
Flórez A, Rosón E, Conde A, González B, García-Doval I, de la Torre C, et al. Toxic epidermal necrolysis secondary to timolol, dorzolamide, and latanoprost eyedrops. J Am Acad Dermatol 2005;53:909-11.
[Google Scholar]
|
| 184. |
Mockenhaupt M, Viboud C, Dunant A, Naldi L, Halevy S, Bouwes Bavinck JN, et al. Stevens-Johnson syndrome and toxic epidermal necrolysis: assessment of medication risks with emphasis on recently marketed drugs. The EuroSCAR-study. J Invest Dermatol 2008;128:35-44.
[Google Scholar]
|
| 185. |
Roujeau JC, Kelly JP, Naldi L, Rzany B, Stern RS, Anderson T, et al. Medication use and the risk of Stevens-Johnson syndrome or toxic epidermal necrolysis. N Engl J Med 1995;333:1600-7.
[Google Scholar]
|
| 186. |
Rzany B, Mockenhaupt M, Norbert Hollander N, Zobel K, Stocker U, Schröder W, et al. Very low risk for ace-inhibitors associated with Stevens-Johnson syndrome and toxic epidermal necrolysis based on prescription data in defined daily doses. J Invest Dermatol 1994;102:619.
[Google Scholar]
|
| 187. |
England JR, England JD. Alopecia and propranolol therapy. Aust Fam Physician 1982;11:225-6.
[Google Scholar]
|
| 188. |
Graeber CW, Lapkin RA. Metoprolol and alopecia. Cutis 1981;28:633-4.
[Google Scholar]
|
| 189. |
Fraunfelder FT, Meyer SM, Menacker SJ. Alopecia possibly secondary to topical ophthalmic beta-blockers. JAMA 1990;263:1493-4.
[Google Scholar]
|
| 190. |
Parker LN, Lifrak ET, Odell WD. Lack of a gonadal or adrenal androgenic mechanism for the hypertrichosis produced by diazoxide, phenytoin and minoxidil. Biochem Pharmacol 1982;31:1948-50.
[Google Scholar]
|
| 191. |
Bublin JG, Thompson DF. Drug-induced hair colour changes. J Clin Pharm Ther 1992;17:297-302.
[Google Scholar]
|
| 192. |
Read GM. Verapamil and hair colour change. Lancet 1991;338:1520.
[Google Scholar]
|
| 193. |
Daniel CR 3rd. Onycholysis: an overview. Semin Dermatol 1991;10:34-40.
[Google Scholar]
|
| 194. |
Femiano F, Lanza A, Buonaiuto C, Gombos F, Rullo R, Festa V, et al. Oral manifestations of adverse drug reactions: guidelines. J Eur Acad Dermatol Venereol 2008;22:681-91.
[Google Scholar]
|
| 195. |
Gómez-Moreno G, Guardia J, Aguilar-Salvatierra A, Cabrera-Ayala M, Maté-Sánchez de-Val JE, Calvo-Guirado JL. Effectiveness of malic acid 1% in patients with xerostomia induced by antihypertensive drugs. Med Oral Patol Oral Cir Bucal 2013;18:e49-55.
[Google Scholar]
|
| 196. |
Madinier I, Berry N, Chichmanian RM. Drug-induced oral ulcerations. Ann Med Interne (Paris) 2000;151:248-54.
[Google Scholar]
|
| 197. |
Aksdal E, Stokke T, Løkken P. Methyldopa – Mouth mucosa ulcerations. Nor Tannlaegeforen Tid 1979;89:504-5.
[Google Scholar]
|
| 198. |
Mikeljevic J, Highet AS. Nicorandil-induced leg ulceration without mucosal involvement. Clin Exp Dermatol 2011;36:372-3.
[Google Scholar]
|
| 199. |
Samarasinghe YP, Cox A, Feher MD. Calcium channel blocker induced gum hypertrophy: no class distinction. Heart 2004;90:16.
[Google Scholar]
|
| 200. |
Agbabiaka TB, Savovic J, Ernst E. Methods for causality assessment of adverse drug reactions: a systematic review. Drug Saf 2008;31:21-37.
[Google Scholar]
|
| 201. |
Lammintausta K, Kortekangas-Savolainen O. The usefulness of skin tests to prove drug hypersensitivity. Br J Dermatol 2005;
[Google Scholar]
|
| 202. |
152:968-74.
[Google Scholar]
|
| 203. |
Goldberg I, Gilburd B, Kravitz MS, Kivity S, Chaim BB, Klein T, et al. A novel system to diagnose cutaneous adverse drug reactions employing the cellscan – Comparison with histamine releasing test and Inf-gamma Releasing Test. Clin Dev Immunol 2005;12:85-90.
[Google Scholar]
|
Fulltext Views
26,853
PDF downloads
5,394





