Translate this page into:
Association of intermediate Nugent Score and bacterial vaginosis with sexually transmitted infections and vulvovaginal candidiasis
Corresponding author: Dr. Sunil Sethi, Vice Chair South East Asia , IUSTI Asia Pacific, Nodal officer, STI Reference, Research & Training Centre & RNTCP Accredited TB Centre, Professor & In-Charge STD, TB & Serology division, Department of Medical Microbiology, PGIMER, Chandigarh, India. sunilsethi10@hotmail.com
-
Received: ,
Accepted: ,
How to cite this article: Sethi S, Yadav R, Sharma N, Dadwal R, Chaudary H, Kaur K, et al. Association of intermediate Nugent Score and bacterial vaginosis with sexually transmitted infections and vulvovaginal candidiasis. Indian J Dermatol Venereol Leprol. 2024;90:296-301. doi: 10.25259/IJDVL_775_2022
Abstract
Background
Bacterial vaginosis is a common vaginal syndrome among females, which leads to significant morbidity and complications, if left untreated. The association of bacterial vaginosis with various sexually transmitted infections has been mentioned in previous literature. However, studies on the intermediate Nugent Score are lacking. This study was planned to examine the association of sexually transmitted infections with the intermediate Nugent Score.
Materials and Methods
The study included was conducted to include females presenting with vaginal discharge, burning micturition, itching, lower abdominal pain and infertility. The Nugent scoring was used to categorize patients into those having normal flora, intermediate or bacterial vaginosis. Conventional and molecular techniques targeting Trichomonas vaginalis, Chlamydia trachomatis, Ureaplasma urealyticum, Mycoplasma hominis, Syphilis, Neisseria gonorrhoeae and vulvovaginal candidiasis were performed.
Results
A total of 3,531 clinical samples were collected from females with a median age of 28.0 years. The number of patients with bacterial vaginosis and intermediate Nugent Score and positive cases were significantly higher in the 21–35 years age group (P < 0.0001). We observed that the likelihood of test results being positive for Trichomonas vaginalis was higher (P < 0.05), as the abnormality of the vaginal flora increased. Mycoplasma hominis was observed to be significantly higher in the intermediate Nugent Score group than the BV-positive patients (0.6 vs 0.2, P = 0.002). The number of vulvovaginal candidiasis cases in both the bacterial vaginosis-negative and bacterial vaginosis-positive groups were nearly the same (9.3 vs 9.8%).
Limitation
Individual follow-up couldn’t be performed on the patients.
Conclusion
We observed that the dysbiosis in vaginal microbiota, with an increase in Nugent scoring, was significantly associated with an increased risk for the acquisition of sexually transmitted infections and vulvovaginal candidiasis.
Keywords
Bacterial vaginosis
Intermediate nugent score
Mycoplasma hominis
Trichomonas vaginalis
Vulvovaginal candidiasis.
Plain Language Summary
This clinical study evaluated the association of bacterial vaginosis and intermediate stage, as per Nugent’s Score, with various sexually transmitted infections and vulvovaginal candidiasis. We observed an incremental increase in the likelihood of acquisition of sexually transmitted infections and vulvovaginal candidiasis with the increase in the abnormality of vaginal flora.
Introduction
Bacterial vaginosis (BV) is a common vaginal syndrome due to the alteration of normal vaginal flora. The vaginal lactobacilli are replaced and dominated by the growth of anaerobes and gram-negative bacteria (Gardnerella vaginalis).1 This leads to disruption of vaginal microflora resulting in grey or white, thin, and malodorous discharge. Worldwide, the prevalence of bacterial vaginosis varies substantially, ranging from 15 to 49%.2–5 It has been associated with complications related to women’s reproductive health and contributes to morbidity among females in developing countries. Traditionally, Nugent scoring is used to categorise vaginal flora as bacterial vaginosis normal, intermediate and positive.6 Previously, many studies have observed association and increased susceptibility of various sexually transmitted infections (STIs) in BV positive and negative patients.7 In a recent systematic review and meta-analysis, a nearly 2-fold higher risk for acquiring Trichomonas vaginalis was observed in bacterial vaginosis-positive patients than in those without bacterial vaginosis.8 Also, concurrent vaginal Candida colonization was independently associated with approximately two-fold increased odds for bacterial vaginosis.9
Studies on intermediate Nugent Score (BV intermediate) are lacking. There is a growing body of literature that vaginal bacterial communities, like lactobacillus, play a pivotal role in preventing colonization and infection by pathogens causing STIs and vulvovaginal candidiasis. Only a few prospective studies have been assessed to see the association of vaginal microbiota with the incidence of STIs and vulvovaginal candidiasis. However, these studies were conducted among high-risk group females, that is, female sex workers or women attending STIs clinics.10,11 Most of these studies have observed the relationship between various STIs and vulvovaginal candidiasis between BV positive and negative patients. As known, the increase in the Nugent Score corresponds to the progression in vaginal dysbiosis and decrease in the lactobacillus population.6 Bacterial vaginosis intermediate has the propensity to progress towards the BV-positive stage. Thus, it is important to study the association of STIs in the BV positive and BV intermediate categories individually. Therefore, this study was conducted to evaluate the relationship between STIs and vulvovaginal candidiasis among female subjects with negative, intermediate and positive bacterial vaginosis categories individually.
Material and Methods
Study design and recruitment
The study was conducted from 1st January 2017 to 31st December 2021 at the regional STIs reference, research and training center of the Postgraduate Institute of Medical Education and Research, Chandigarh, which caters 14 peripheral centers in Chandigarh. A total of 3,531 clinical samples were collected from females (16–65 years of age) with vaginitis. Females presenting with any of the following chief complaints such as vaginal discharge, burning micturition, itching, lower abdominal pain, or infertility were enrolled. Two vaginal swabs were collected by the trained healthcare professional using FLOQSwabsTM (Copan, Italy) during routine pelvic examinations. Females were excluded if they had received antibiotics or antifungals in the preceding week or were menstruating or pregnant. Females with genital tuberculosis and malignancies were also excluded. The study was approved by the Institutional Ethics Committees, Postgraduate Institute of Medical Education and Research, Chandigarh (Ethics approval no. INT/IEC/2019/002222).
Microbiologic Analysis
Bacterial vaginosis: The vaginal fluid was smeared on a glass slide and stained with gram stain and reported for the presence of bacterial vaginosis, in accordance with Nugent scoring criteria.6 Bacterial vaginosis was diagnosed if the score was 7–10; a score of 4–6 indicated intermediate vaginal flora; and a score of 0–3 indicated normal vaginal flora.
Test for Trichomonas vaginalis: For Trichomonas vaginalis culture, vaginal swabs were inoculated in Diamond’s media and incubated at 37°C. The media were examined every second day for 1 week for the presence of motile trichomonads. The tvk3 and tvk7 primers were used as their targets, specifically amplifying a 261 bp sequence of the 18S SS-rRNA gene segments of Trichomonas vaginalis. The conventional polymerase chain reaction was performed as per the protocol.12
Tests for Neisseria gonorrhoeae: Endocervical samples were streaked on a chocolate medium. Identification was confirmed by gram stain examination, superoxol (10% hydrogen peroxide), rapid carbohydrate utilization test and a polymerase chain reaction of the porA and opa gene.13
Test for Chlamydia trachomatis, Mycoplasma hominis and Ureaplasma urealyticum: The quantitative polymerase chain reaction for the detection of C. trachomatis, Ureaplasma urealyticum and Mycoplasma hominis, was performed using the primers from previous literature.14
Test for syphilis: The Venereal Disease Research Laboratory (VDRL) test was performed as per the World Health Organization guidelines.15 The treponema pallidum hemagglutination (TPHA) test was performed by Plasmatec kit (Novacyt Group, United Kingdom) as per the manufacturer’s guideline.16
Yeast identification: It was done by matrix-assisted laser desorption/ionization-time of flight mass spectrometry (Bruker Daltonics MALDI Biotyper, Germany).
Data analysis and statistics
The data was entered in Statistical Package for the Social Sciences 26.0 for Mac OS (SPSS, Inc., Chicago, IL). A P < 0.05 was used analyse the data.
Results
Characteristics of the study population
The age of females in the present study ranged from 15 to 50 years, with a median age of 28.0 years (interquartile range: 10) [Figure 1]. Based on the Nugent scoring, number of patients with positive, intermediate, and negative scores for BV were 479 (13.6%), 837 (23.7%) and 2215 (62.7%), respectively. Among the subjects with BV, 7.7% (37 of 479) were infected with one of the two sexually transmitted organisms, compared with an infection rate of 6.2% (52 of 837) in patients with BV intermediate and 2.8% (64 of 2215) for subjects without BV.
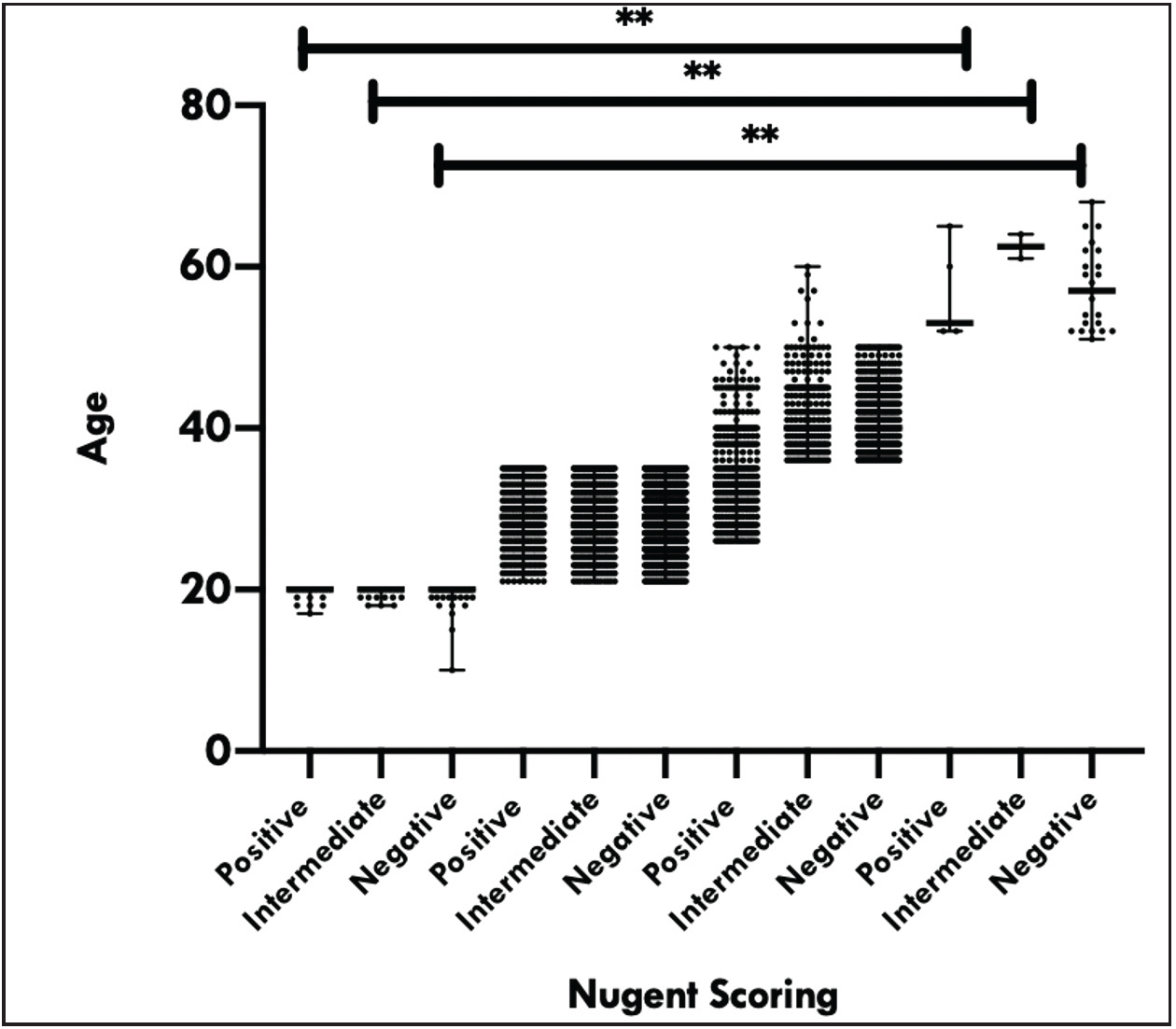
- Relation of various categories of Nugent scoring with different age groups
Association of age groups with bacterial vaginosis stages
The BV-positive females were mostly from 21–35 age group, followed by the age group of 36–50 years [Figure 1]. The number of positive BV cases was significantly higher in 21–35 years age group (P < 0.0001). Similarly, the number of BV intermediate patients was significantly higher in 21–35 years age group (P < 0.0001) than in other age groups.
Association of bacterial vaginosis stages with clinical symptoms
The frequency of cervicitis and discharge was significantly more in patients with BV intermediate category than in negative and positive BV groups (10.9 vs 7.1 vs 7.7%, P = 0.003).
Association of bacterial vaginosis stages with sexually transmitted infections
Among the subjects with BV, 25 (31.3%) were infected with Trichomonas vaginalis [Table 1]. We found a significant difference in patients with and without BV for Trichomonas vaginalis acquisition (P < 0.05). Patients with BV were three times more likely to be infected with TV than those without BV (95% confidence interval, 1.8–4.8) [Table 2]. Likewise, patients with BV were significantly more positive for Candida species than patients without BV (odds ratio, 1.2; 95% confidence interval, 0.85–1.630) [Table 2]. The likelihood of the test results being positive for Trichomonas vaginalis was significantly higher (P < 0.05) as the abnormality of the vaginal flora increased. Mycoplasma hominis was observed to be significantly higher in BV intermediate than BV positive patients (0.6 vs 0.2, P = 0.002) [Table 1]. Also, concurrent Trichomonas vaginalis and Mycoplasma hominis were found in 5 of 6 of the patients. Although not significant, an increasing trend was observed in Ureaplasma urealyticum infection in BV patients, than in BV normal and BV intermediate categories (P = 0.064) [Table 1]. Neisseria gonorrhoeae and Chlamydia trachomatis were present in similar frequency among all the categories.
| Negative (2215) n (%) | Intermediate (837) n (%) | Bacterial vaginosis positive (479) n (%) | P-value | |
|---|---|---|---|---|
| Symptoms | ||||
| Cervicitis | 159 (7.1) | 92 (10.9) | 37 (7.7) | 0.003 |
| Presence of discharge | 1278 (57.7) | 552 (65.9) | 300 (62.6) | 0.000 |
| Infertility | 354 (15.9) | 130 (15.5) | 70 (14.6) | 0.749 |
| Burning micturition | 29 (1.3) | 10 (1.2) | 4 (0.84) | 0.690 |
| Itching | 81 (3.6) | 29 (3.4) | 20 (4.2) | 0.801 |
| Abdominal pain | 68 (3.0) | 15 (1.8) | 13 (2.7) | 0.153 |
| Infections | ||||
| Trichomonas vaginalis | 23 (1) | 32 (3.8) | 25 (5.2) | 0.000 |
| Chlamydia trachomatis | 3 (0.1) | 1 (0.11) | 0 | 0.725 |
| Ureaplasma urealyticum | 4 (0.2) | 3 (0.3) | 4 (0.8) | 0.064 |
| Mycoplasma hominis | 0 | 5 (0. 6) | 1 (0.2) | 0.002 |
| Syphilis | 28 (1.3) | 11 (1.3) | 6 (1.3) | 0.993 |
| Neisseria gonorrhoeae | 6 (0.3) | 2 (0.2) | 1 (0.2) | 0.965 |
| Vulvovaginal candidiasis | 180 (8.2) | 78 (9.3) | 47 (9.8) | 0.356 |
BV: bacterial vaginosis
| Characteristics | Bacterial vaginosis | Odd’s ratio (95% confidence interval) | P | |
|---|---|---|---|---|
| Present n (%) | Absent n (%) | |||
| Presence of discharge | 300 (14.1) | 1830 (85.9) | 1.12 (0.92–1.36) | 0.270 |
| Infertility | 70 (12.6) | 484 (87.4) | 0.91 (0.69–1.19) | 0.543 |
| Burning micturition | 4 (9.3) | 39 (90.7) | 0.65 (0.23–1.83) | 0.288 |
| Itching | 20 (15.4) | 110 (84.6) | 1.16 (0.72–1.89) | 0.305 |
| Abdominal pain | 13 (13.5) | 83 (86.5) | 0.99 (0.55–1.81) | 0.571 |
| Cervicitis | 37 (12.8) | 251 (87.2) | 0.94 (0.65–1.34) | 0.788 |
| Trichomonas vaginalis | 25 (31.3) | 55 (68.8) | 3.01 (1.85–4.86) | 0.000 |
| Chlamydia trachomati | 0 (0.0) | 4 (100) | 0.99 (0.99–1.00) | 1.000 |
| Ureaplasma urealyticum | 4 (36.4) | 7 (63.6) | 3.66 (1.07–12.56) | 0.50 |
| Mycoplasma hominis | 1 (16.7) | 5 (83.3) | 1.27 (0.15–10.94) | 0.583 |
| Syphilis | 6 (13.3) | 39 (86.7) | 0.98 (0.43–2.33) | 1.00 |
| Neisseria gonorrhoeae | 1 (11.1) | 8 (88.9) | 0.79 (0.09–6.38) | 1.00 |
| Vulvovaginal candidiasis | 47 (15.4) | 258 (84.6) | 1.17 (0.85–1.64) | 0.336 |
Association of bacterial vaginosis with vulvovaginal candidiasis
Of all the subjects with BV, 14 (15.4%) women had vulvovaginal candidiasis. The BV patients were 1.2 times more likely to be infected with yeast infection than were subjects without BV (95% confidence interval, 0.850–1.634). The number of vulvovaginal candidiasis cases in both BV intermediate and BV positive was nearly the same (9.3 vs 9.8%). However, the difference in vulvovaginal candidiasis between BV positive and negative groups was not significant (P = 0.336). Figure 2 depicts the distribution of various yeast species among patients with different Nugent scores. Chlamydia trachomatis was the most common yeast isolated overall, followed by Candida glabrata (195 vs 85) [Figure 2]. Other less common yeasts were Chlamydia trachomatis, Candida krusei, Candida kefyr, Candida parapsilosis and Candida lambica.
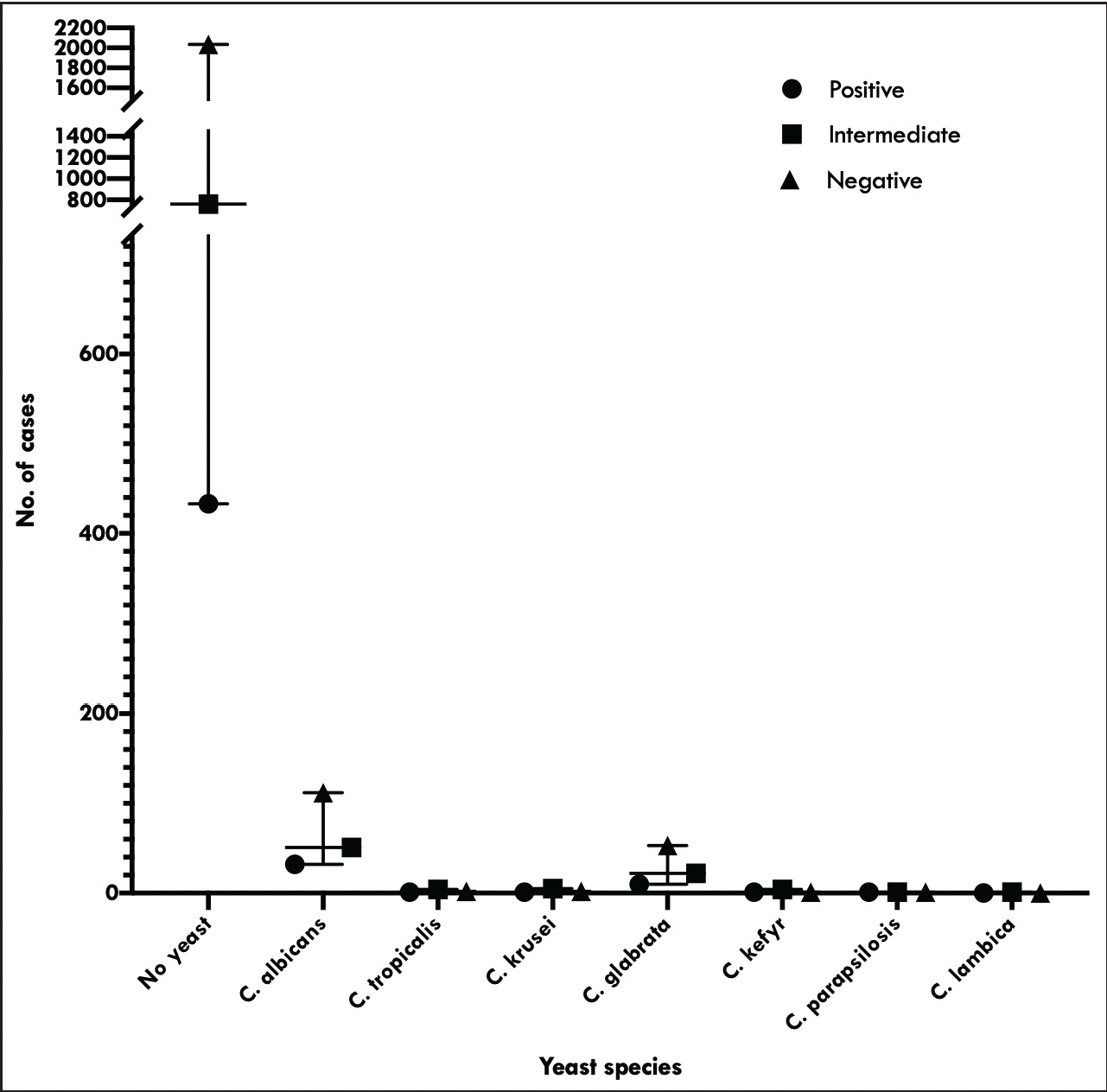
- The distribution of various yeast species among patients with different Nugent Scores viz; normal, intermediate and bacterial vaginosis
Discussion
This study represents a large study population that provides evidence of the association of BV positive and BV intermediate with sexually transmitted infections and vulvovaginal candidiasis. The prevalence of BV in the current study was 13.6%, which is nearly similar to another study from the South India where the prevalence was 15.4%.2 However, a higher rate of BV has been reported in various other parts of the world, ranging from 29 to 49%.3–5
We observed an incremental increase (3.8–5.2%) in the likelihood of acquisition of Trichomonas vaginalis infection with an increase in the abnormality of vaginal flora. Similar findings were also observed by Brotman et al., where the intermediate state was associated with a 1.5–2-fold increased risk for Trichomonas vaginalis infection.17 These above findings are suggestive that females without BV but with an altered vaginal flora may pose a risk for STIs.24 Although epidemiological data suggest a strong association of Trichomonas vaginalis infection with BV, the prevalence of these infections is probably underestimated since both can be asymptomatic or clinically present with common symptoms of vaginal discharge.18 Rathod et al. observed a four to nine-fold increased risk of Trichomonas vaginalis infection among sexually active females with abnormal vaginal flora, suggesting a causal role of altered microbiota on trichomonad infection.19 In in vitro experiments, Hinderfeld and Simoes-Barbosa observed that biofilm produced by dysbiotic bacterias increased the adhesion of the protozoa to host cells. Thus, it is plausible that BV intermediate alters the vaginal ecology and facilitates the acquisition of STIs.
Secondly, we observed a higher number of Mycoplasma hominis cases in the BV intermediate category than BV positive cases (P = 0.002). On the contrary, Cox et al., observed a significantly higher presence of Mycoplasma hominis in BV positive (60.7%) patients compared to BV intermediate and nonbacterial vaginosis (36.4 and 8.8 %, P < 0.001) stages.20 Both the BV positive and BV intermediate stages are characterized by decreased protective lactobacilli.21 These findings suggest BV intermediate has unique epidemiology and represents unique taxa of the bacterial community, which needs to be defined by further studies.21
Mycoplasma hominis has been found in symptomatic women as well as healthy females. Various studies have observed its association with altered vaginal flora, including BV positive and Trichomonas vaginalis infection. Belkum et al. observed the highest prevalence of Mycoplasma hominis prevalence in the groups of patients infected with Trichomonas vaginalis or those who were BV positive (71 and 38%, respectively).22 This was also observed in the present study, where the maximum number of cases (5 of 6) had concurrent Trichomonas vaginalis and Mycoplasma hominis infection. We did not observe an increase in symptoms once Mycoplasma hominis infection was detected along with Trichomonas vaginalis. However, the question remains elusive, whether in BV, the Trichomonas vaginalis predisposes to Mycoplasma hominis infection or if this is merely an association. In one study, a symbiotic association between Trichomonas vaginalis and Mycoplasma hominis was observed to influence the host-microbes interactions, which is detrimental to their host but beneficial to both microbial partners during infections.23 However, further research is required to study the association between these STIs.
In the current study, concurrent vaginal Candida colonisation was associated with approximately one-fold increased odds of BV. Although not significant, the higher trend of vulvovaginal candidiasis was present in both BV intermediate and BV positive stages [Table 1]. In India, Rathod and co-workers found a positive association between women clinically diagnosed with BV positive and prevalence of vulvovaginal candidiasis (prevalence bacterial vaginosis: bacterial vaginosis normal 12%:6.5%).24 As both BV positive and vulvovaginal candidiasis have overlapping clinical presentation of vaginal discharge, the misclassification between vulvovaginal candidiasis and clinically defined BV is most likely possible.24 The high prevalence of yeast infection/colonisation in BV intermediate may predispose these females to symptomatic vulvovaginal candidiasis, thus leading to repeated visits to hospitals. Also, a significant number of females who are BV positive have yeast colonisation in the vaginal ecosystem. Hence the failure of symptom resolution in patients with targeted therapy to treat BV can be due to the development of vulvovaginal candidiasis from antibiotic exposure or intrinsic failure of the therapy.25 Thus, the identification of patients with concurrent BV and vulvovaginal candidiasis is important due to a major concern of recurrence of symptoms in patients. Lopez et al., observed concurrent BV and vulvovaginal candidiasis in 34% of cases and 29% of patients had a history of recurrent BV.26 The lack of specificity of clinical signs and symptoms of vaginitis and high rate of recurrence mandate clinical and microbiological examination to determine a specific diagnosis. Failure to appreciate the vulvovaginal candidiasis in BV-positive patients leads to inappropriate therapy and increases the rate of recurrence. In accordance with previous studies, Candida albicans was the most frequently isolated species; however, we also observed a high prevalence rate of nonalbicans Candida species.27,28 Thus, yeast identification, antifungal susceptibility, and continuous epidemiological surveys to measure changes in species distribution from Candida albicans to non-albicans Candida species are important.
A healthy vaginal microbiome plays a pivotal role in protecting the female genital tract against various STIs and vulvovaginal candidiasis. The alteration of host defence against infection due to a decrease in the protective lactobacillus-deficient environment leads to increased susceptibility to STIs due to the presence of BV associated organisms and their metabolites. Further release of microbial products alters the vaginal microenvironment.
The major strength of the present study is simultaneous reporting of Nugent Score, cultures, and detection of STIs. While most of the previous literature has been majorly defined in high-risk population, our study assessed females presenting for routine health care, thus providing a panoramic view of the STIs in BV intermediate.
Taking this into consideration, future research should focus on whether intervention should be recommended for females who present with BV intermediate stage. The metagenomics and transcriptomics of vaginal flora in healthy and BV females can facilitate our understanding of the interaction of microbial communities. The knowledge of these communities will assist in the development of interventions that can drive the vaginal microbiota toward a healthier state.
Ethical Approval
The study was approved by the Institutional Ethics Committees, Postgraduate Institute of Medical Education and Research, Chandigarh (Ethics approval no. INT/IEC/2019/002222)
Declaration of patient consent
Institutional Review Board (IRB) permission was obtained for the study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Bacterial vaginosis: A review of treatment, recurrence, and disparities. J Nurse Practition.. 2019;15:420-3.
- [Google Scholar]
- Prevalence and correlates of bacterial vaginosis among young women of reproductive age in Mysore, India. Indian J Med Microbiol. 2008;26:132-7.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- The prevalence of bacterial vaginosis in the United States, 2001–2004; associations with symptoms, sexual behaviors, and reproductive health. Sex Transm Dis. 2007;34:864-9.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence of and risk factors for bacterial vaginosis among women of reproductive age attending cervical screening in southeastern Brazil. Int J Gynaecol Obstet. 2015;131:137-41.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence of bacterial vaginosis and associated risk factors among women complaining of genital tract infection. Int J Microbiol. 2017;2017:4919404.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J Clin Microbiol. 1991;29:297-1.
- [CrossRef] [PubMed] [Google Scholar]
- Bertini M. Bacterial Vaginosis and Sexually Transmitted Diseases: Relationship and Management [Internet]. Fundamentals of Sexually Transmitted Infections. InTech; 2017. Available from: http://dx.doi.org/10.5772/intechopen.69258
- Bacterial vaginosis and its association with incident trichomonas vaginalis infections: a systematic review and meta-analysis. Sex Transm Dis. 2021;48:e192-201.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Prevalence, risk factors and adverse pregnancy outcomes of second trimester bacterial vaginosis among pregnant women in Bukavu, Democratic Republic of the Congo. PLoS One. 2021;16:e0257939.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Vaginal lactobacilli, microbial flora, and risk of human immunodeficiency virus type 1 and sexually transmitted disease acquisition. J Infect Dis. 1999;180:1863-8.
- [CrossRef] [PubMed] [Google Scholar]
- Abnormal vaginal flora as a biological risk factor for acquisition of hiv infection and sexually transmitted diseases. J Infect Dis.. 2005;192:1315-7.
- [CrossRef] [PubMed] [Google Scholar]
- Trichomonas vaginalis: repeated DNA target for highly sensitive and specific polymerase chain reaction diagnosis. Cell mol biol (Noisy-le-Grand, France). 1994;40:819-31.
- [Google Scholar]
- Evaluation of strategies for confirming Neisseria gonorrhoeae nucleic acid amplification tests. J Med Microbiol. 2011;60:909-12.
- [CrossRef] [PubMed] [Google Scholar]
- Development of real-time PCR for the differential detection and quantification of Ureaplasma urealyticum and Ureaplasma parvum. J Microbiol Meth.. 2005;60:13-19.
- [Google Scholar]
- Ballard R, Ison C, Lewis D, Ndowa F, Peeling R, eds. Laboratory diagnosis of sexually transmitted infections including human immunodeficiency virus [Monograph on the Internet]. World Health Organisation; 2013.
- https://plasmatec.co.uk/syphilis-serology-tests/#tpha (accessed February 7, 2022).
- Bacterial vaginosis assessed by gram stain and diminished colonization resistance to incident gonococcal, chlamydial, and trichomonal genital infection. J Infect Dis. 2010;202:1907-15.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Trichomonas vaginalis infection in symbiosis with Trichomonasvirus and Mycoplasma. Res Microbiol. 2017;168:882-91.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Bacterial vaginosis and risk for trichomonas vaginalis infection: A longitudinal analysis. Sex Transmit Dis. 2011;38:882-6.
- [Google Scholar]
- Mycoplasma hominis and Gardnerella vaginalis display a significant synergistic relationship in bacterial vaginosis. Eur J Clin Microbiol Infecti Dis. 2016;35:481-7.
- [Google Scholar]
- Vaginal microbiome of reproductive-age women. Proc Natl Acad Sci USA. 2011;108 Suppl 1:4680-7.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- A clinical study on the association of Trichomonas vaginalis and Mycoplasma hominis infections in women attending a sexually transmitted disease (STD) outpatient clinic. FEMS Immunol Med Microbiol. 2001;32:27-32.
- [CrossRef] [PubMed] [Google Scholar]
- Symbiotic association with Mycoplasma hominis can influence growth rate, ATP production, cytolysis and inflammatory response of Trichomonas vaginalis. Front Microbiol. 2016;7:953.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Epidemiologic features of vulvovaginal candidiasis among reproductive-age women in India. Infect Dis Obstet Gynecol. 2012;2012:859071.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Prevalence of bacterial vaginosis and vulvovaginal candidiasis mixed infection in a Southeastern American STD clinic. Sex Transmit Dis. 2011;38:672-4.
- [Google Scholar]
- Vulvovaginal candidiasis complicating recurrent bacterial vaginosis. Sex transmit dis. 1990;17:51-53.
- [Google Scholar]
- Epidemiology and antifungal susceptibility patterns of candida isolates from Greek women with vulvovaginal candidiasis. Mycoses. 2019;62:692-7.
- [CrossRef] [PubMed] [Google Scholar]
- Species distribution and antifungal susceptibility profiles of isolates from women with nonrecurrent and recurrent vulvovaginal candidiasis. Microb Drug Resist. 2021;27:1087-95.
- [CrossRef] [PubMed] [Google Scholar]






