Translate this page into:
Atypical presentations of eosinophilic fasciitis
2 Department of Rheumatology, Marmara University School of Medicine, Istanbul, Turkey
3 Department of Pathology, Cerrahpasa Medical Faculty, Istanbul University, Istanbul, Turkey
4 Department of Radiology, Marmara University School of Medicine, Istanbul, Turkey
5 Department of Hematology, Marmara University School of Medicine, Istanbul, Turkey
Correspondence Address:
Andac Salman
Department of Dermatology, Marmara University Pendik Research and Training Hospital, Fevzi Cakmak Mah, Mimar Sinan Cad., No. 41, Pendik, 34899 Istanbul
Turkey
| How to cite this article: Ergun T, Seckin D, Salman A, Ocak ES, Yucelten AD, Direskeneli H, Demirkesen C, Ekinci G, Bayik M. Atypical presentations of eosinophilic fasciitis. Indian J Dermatol Venereol Leprol 2016;82:47-52 |
Abstract
Eosinophilic fasciitis is an uncommon connective tissue disease that may mimic and overlap with other sclerosing disorders such as morphea and lichen sclerosus. Herein, we report four patients (two men and two women, aged 16-64 yeas) with eosinophilic fasciitis. There was overlap with both morphea and lichen sclerosus in 2 patients and with morphoea alone in 1 patient. Magnetic resonance imaging (MRI) was used for diagnosis in three patients and for assessing treatment response in one patient. Eosinophilic fasciitis may co-exist with morhoea and lichen sclerosus. In view of the overlapping clinical and histopathological features of these disorders, MRI may be helful in delineating the conditions by detecting involvement of fascia.Introduction
Eosinophilic fasciitis is a rare sclerosing disease with a wide clinical spectrum varying from a limited disease with a benign course where cure is possible to a more generalized disease with organ involvement and poor response to treatment.[1],[2]
We present four cases of eosinophilic fasciitis with unusual features to highlight a possible overlap with morphea and lichen sclerosus and also highlight the importance of magnetic resonance imaging which can be a diagnostic tool.
Case Reports
Case 1
A 64-year-old woman with generalized stiffness of the skin, weight loss, fatigue and abdominal distention was referred to dermatology from the hematology clinic where she was diagnosed as hypereosinophilic syndrome and was being treated with systemic steroids for 13 months followed by hydroxyurea without any response.
Physical examination revealed diffuse sclerosis of skin more pronounced on lower legs, abdomen and forearms sparing the face, hands and feet. She also had ivory-colored sclerotic plaques on chest, forearms and proximal legs, some of them surrounded by a violaceous halo. Puckering of skin, peau d'orange appearance as well as furrowing were striking on the thighs [Figure - 1]. Raynaud's phenomenon was absent, and nailfold capillaroscopy was normal.
 |
| Figure 1: Case 1: Peau d'orange appearance as well as furrowing in proximal legs |
Relevant laboratory findings were antinuclear antibody positivity (1:80), peripheral blood eosinophilia (21% of white blood cells), high erythrocyte sedimentation rate and hypergammaglobulinemia [Table - 1]. Extractable nuclear antigens, rheumatoid factor, and Lyme antibodies were negative. Bone marrow biopsy had revealed marked eosinophilia. An extensive search for malignancy failed to show any underlying malignancy.
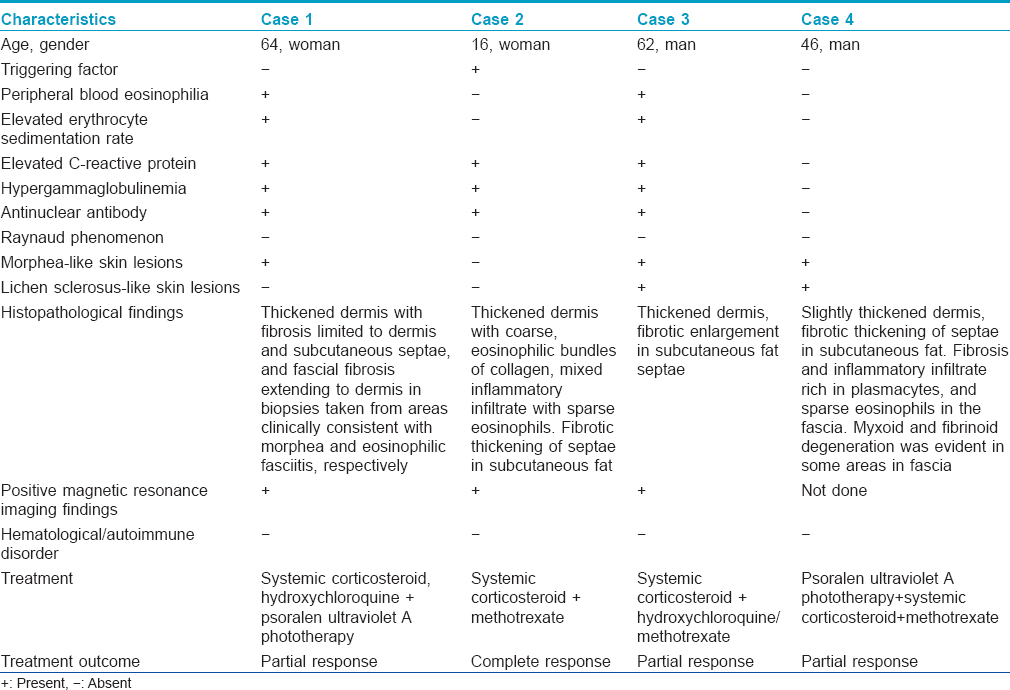
A punch biopsy obtained from the lesion clinically suggestive of morphea revealed dermal fibrosis whereas biopsy from an area with diffuse sclerosis of the lower leg revealed fascial fibrosis extending to dermis with a mixed inflammatory infiltrate containing eosinophils [Table - 1]. Magnetic resonance imaging of the lower extremity revealed thickening and increased signal intensity within the fascia and fascial enhancement after contrast administration. There was an edema-like signal within the muscle fibers adjacent to fascia and overlying subcutaneous tissue [Figure - 2]a,[Figure - 2]b,[Figure - 2]c.
 |
| Figure 2: Case 1: (a) Axial fast spin echo T1-weighted (b) and axial fat suppressed fast spin echo T2-weighted images of right lower extremity show fascial thickening and increased signal intensity, respectively. (c) Contrast-enhanced axial fat suppressed fast spin echo T1-weighted image shows fascial enhancement |
Accordingly, she was diagnosed as eosinophilic fasciitis with generalized morphea and treatment with methylprednisolone 0.8 mg/kg/day was re-initiated. Due to the development of diabetes mellitus and lack of response, steroids were tapered and treatment with hydroxychloroquine and psoralen and ultraviolet A therapy (PUVA) were initiated. At the end of 60 psoralen and ultraviolet A (PUVA) sessions, plaques of morphea resolved and skin stiffness improved partially. Post-treatment magnetic resonance imaging revealed minimal improvement. Treatment was subsequently stopped and she had no clinical deterioration over a 5 year period of follow up. A recent work-up revealed no autoimmune disorder or malignancy.
Case 2
A 16-year-old otherwise healthy woman presented with a 2-month history of difficulty in opening her hands. She was practicing violin and had not exercised vigorously. Physical examination revealed stiffness of both arms and neck with prayer sign in both hands. Fingers were not affected and Raynaud's phenomenon was absent.
Laboratory tests revealed a high C-reactive protein and hypergammaglobulinemia. Her eosinophil count was 400/mm 3. Antinuclear antibody was positive in low titer (1:100) but extractable nuclear antigens were negative [Table - 1].
Histopathological findings are shown in [Table - 1]. Magnetic resonance imaging of upper extremity revealed marked thickening and increased signal intensity within the fascia and prominent fascial enhancement after contrast administration. An edema-like signal was seen within the muscle fibers adjacent to the fascia. No signal abnormality was seen within the overlying subcutaneous tissue [Figure - 3]a and [Figure - 3]b.
 |
| Figure 3: Case 2: (a) Axial fast spin echo T1-weighted (b) and contrast–enhanced fat suppressed fast spin echo T1-weighted images of upper extremity show increased signal intensity and thickening of the fascia, respectively |
She was diagnosed as eosinophilic fasciitis and treated with methylprednisolone 0.8 mg/kg/day and methotrexate 7.5 mg/week. At the end of 15 months of treatment, skin findings resolved completely and she remained disease free over 12 months of follow-up.
Case 3
A 62-year-old man presented with a 6-week history of wood-like stiffness of skin on the extremities and trunk, myalgias and muscle weakness. His medications included bisoprolol, valsartan, hydrochlorothiazide, trimetazidine, isosorbide mononitrate, and acetylsalicylic acid which were being used for coronary artery disease and hypertension.
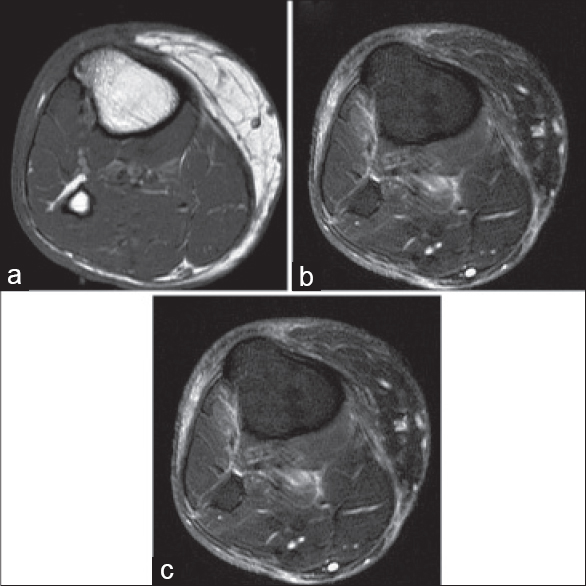
Physical examination revealed woody induration and edema of forearms and legs and purple-gray patches with occasional sclerotic, hypopigmented centers on the lateral aspects of trunk, shoulders, and inguinal region [Figure - 4]. Range of motion of the elbows and knees were limited. Hands and feet were spared.
 |
| Figure 4: Case 3: Generalized morphea lesions on the lateral aspect of the trunk |
He had peripheral eosinophilia (20%, 2700/mm 3), elevated erythrocyte sedimentation rate of 48 mm/h, hypergammaglobulinemia, elevated serum creatinine and antinuclear antibody positivity (1:80). Extractable nuclear antigens profile was negative [Table - 1].
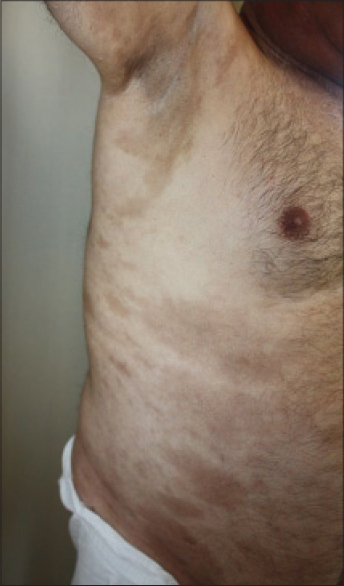
Histopathological examination of the purple-gray, sclerotic patches revealed dermal fibrosis [Table - 1]. Magnetic resonance imaging examination of lower extremity revealed prominent thickening and increased signal intensity within the fascia. An edema-like signal was seen within both the muscle fibers adjacent to the fascia and the overlying subcutaneous tissue [Figure - 5]a and [Figure - 5]b. Eosinophilia-myalgia syndrome was excluded through the absence of muscle cramps, pulmonary symptoms, skin rash, neurological symptoms as well as histopathological and magnetic resonance imaging findings.
 |
| Figure 5: Case 3: (a and b) Fat suppressed fast spin echo T2-weighted image of lower extremity shows increased signal intensity and thickening of the fascia |
Based on these findings, he was diagnosed as eosinophilic fasciitis and morphea-lichen sclerosus overlap. Treatment with methylprednisolone, 48 mg/day and hydroxychloroquine, 400 mg/day was commenced. After 2 weeks of treatment, stiffness of the skin improved slightly and peripheral blood eosinophilia returned to normal (100/mm 3). After 2 months, hydroxychloroquine treatment was stopped and methotrexate 5 mg weekly was added as adjuvant treatment. Methylprednisolone treatment was tapered and stopped at the end of 12 months and the patient is still on treatment with a moderate response.
Case 4
A 46-year-old man presented with an 8-year history of stiffness and swelling of the legs and forearms worsening over the last few months. He had been diagnosed as scleredema and eosinophilic fasciitis in another center. His prior treatments included psoralen and ultraviolet A (PUVA) phototherapy and systemic prednisolone which had led to remission of his complaints. Physical examination revealed stiffness, sclerosis of the forearms and legs sparing the digits and feet as well as sclerotic, centrally ivory-colored patches on the flexor aspects of forearms, legs and ill-defined purple-gray patches on the antero-lateral aspects of trunk [Figure - 6]. Laboratory tests including complete blood count, peripheral blood smear, basic biochemical tests, C-reactive protein, erythrocyte sedimentation rate, antinuclear antibody, and extractable nuclear antigens profile revealed no abnormalities [Table - 1].
 |
| Figure 6: Case 4: Lichen sclerosus-like lesions on the medial aspect of arm |
Histopathological examination revealed fibrotic septal thickening in the subcutaneous fat and fibrosis in the fascia as well as a mixed infiltrate, rich in eosinophils [Figure - 7].
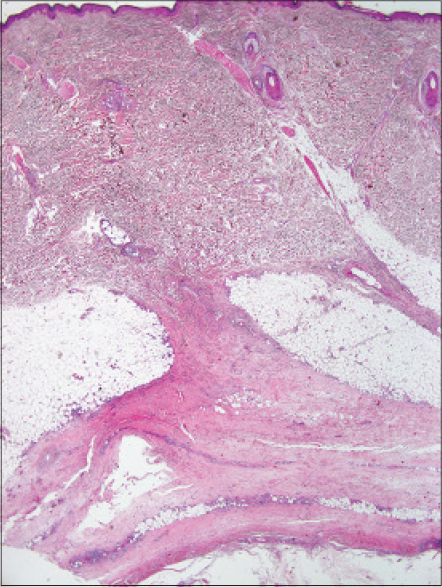 |
| Figure 7: Case 4: Thickening of the septa in subcutaneous fat and prominent fibrotic change in the fascia (biopsy taken from the lesion on the leg clinically resembling eosinophilic fasciitis) (H and E, ×20) |
Based on the clinical and histopathological findings, a diagnosis of eosinophilic fasciitis, morphea and lichen sclerosus overlap was made. He was treated with methotrexate, 7.5 mg/week. After 6 weeks of treatment, stiffness and extent of lesions were improved. He has stable disease after 12 months of methotrexate treatment.
Discussion
We report four cases of eosinophilic fasciitis, two having overlap with both morphea and lichen sclerosus and one with morphea. Magnetic resonance imaging has been used both as a diagnostic tool and in follow-up. Eosinophilic fasciitis is a rare autoimmune disease mimicking scleroderma. Its characteristic findings are sudden-onset erythema, edema in the early phase and symmetrical woody induration of distal extremities later.[1],[2] It is triggered by strenuous exercise and trauma in at least 66% of cases which are hypothesized to induce the antigenicity of the fascia and subcutis.[3],[4] Arthropod bites, borreliosis, Mycoplasma arginini infection and drugs such as simvastatin, atorvastatin, ramipril, and phenytoin are among other triggering factors.[5],[6] Other than our second case having hobby-related overuse of hands, none of our patients had noted any triggering factors.
While eosinophilic fasciitis may be associated with several hematological and autoimmune diseases, none of our patients developed such disorders during the follow-up period ranging from 1 to 5 years.[7] Nearly, a third of patients with eosinophilic fasciitis are reported to have an association with morphea, either preceding or following the onset of the latter.[3],[8],[9] Coexistence of lichen sclerosus and localized scleroderma have also been reported.[10],[11] Despite the possibility of two fibrosing disorders occurring in the same patient, we were unable to find any published reports in English that described coexistent eosinophilic fasciitis, morphea, and lichen sclerosus as was noted in our last two cases. Although not reported previously, the coexistence of these three disorders is not surprising as all are fibrosing in nature and the same stimulus may trigger these different disorders through similar inflammatory pathways.
Our second case is an adolescent, and eosinophilic fasciitis is extremely rare during childhood.[12] Pediatric eosinophilic fasciitis shows a female predominance, a higher incidence of hand involvement, lower incidence of an associated arthritis or hematological disorder and a less favorable course with higher risk of residual fibrosis.[13] Our patient was a female with hand and arm involvement with no evidence of hematological disease, consistent with the literature. However, her disease had a favorable course, completely responding to systemic steroids and methotrexate.
The differentiation of eosinophilic fasciitis from deep morphea or overlapping cases with morphea or scleroderma may be problematic. Overlapping features of eosinophilic fasciitis and deep morphea are involvement of subcutaneous tissue, fascia, muscles, eosinophilia and positive antinuclear antibody and association with systemic sclerosis.[14] The features favoring the diagnosis of eosinophilic fasciitis are venous furrowing, prayer sign, the absence of sclerodactyly and Raynaud's phenomenon, prominent eosinophilia, hypergammaglobulinemia and the absence of nailfold capillary changes. Although eosinophilic fasciitis, morphea and lichen sclerosus are considered different entities both clinically and histopathologically, in view of their common features, one cannot exclude the possibility of these being various manifestations of the same disease.[15],[16] As the histopathological findings of these disorders may overlap to some extent, depending on histopathological examination alone for differential diagnosis may cause difficulties. Consequently, findings of clinical, histopathological and imaging studies should all be evaluated together for precise diagnosis.
The standard diagnostic test for eosinophilic fasciitis is histopathological examination which shows thickening of fascia with sclerosis and occasionally an inflammatory polymorphic infiltrate with varying numbers of eosinophils which can spread to the muscle fibers.[17] Considering the drawbacks of histopathological examination and the validity of magnetic resonance imaging, histopathological examination for confirming the diagnosis may be unnecessary in pediatric cases and also in patients who decline a biopsy. Although magnetic resonance imaging is more expensive, it has several advantages in being non-invasive, rapid, sensitive and devoid of surgical complications. Furthermore, it may provide dermatologists an opportunity to assess improvement objectively. In eosinophilic fasciitis, magnetic resonance imaging images show a thickened deep fasciae on T1-weighted sequences and relatively increased signal intensity greater than that of muscle on fat-suppressed or fat-saturated T2-weighted sequences.[18],[19],[20] Although magnetic resonance imaging findings cannot differentiate eosinophilic fasciitis from other causes of fasciitis, in cases with characteristic clinical, laboratory and magnetic resonance imaging findings, all of the disorders in the differential diagnosis can be excluded. In addition, magnetic resonance imaging can also be used for evaluation of treatment response as imaging findings are consistent with clinical improvement.[18],[19],[20] We used magnetic resonance imaging in three of our cases for diagnosis and in the first case for assessing therapeutic response.
Although high-dose corticosteroids, the gold standard of eosinophilic fasciitis treatment, are reported to be effective in up to 70% of cases, treatment should be individualized. Three of our patients showed a partial/complete response to corticosteroid treatment and all required adjuvant modalities including hydroxychloroquine, methotrexate, and psoralen and ultraviolet A (PUVA).
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Shulman LE. Diffuse fasciitis with hypergammaglobulinemia and eosinophilia: A new syndrome? J Rheumatol 1984;11:569-70.
[Google Scholar]
|
| 2. |
Bischoff L, Derk CT. Eosinophilic fasciitis: Demographics, disease pattern and response to treatment: Report of 12 cases and review of the literature. Int J Dermatol 2008;47:29-35.
[Google Scholar]
|
| 3. |
Lakhanpal S, Ginsburg WW, Michet CJ, Doyle JA, Moore SB. Eosinophilic fasciitis: Clinical spectrum and therapeutic response in 52 cases. Semin Arthritis Rheum 1988;17:221-31.
[Google Scholar]
|
| 4. |
Blaser KU, Steiger U, Würsch A, Speck B. Eosinophilic fasciitis with aplastic anemia and Hashimoto's thyroiditis. Review of the literature and report of a typical example. Schweiz Med Wochenschr 1989;119:1899-906.
[Google Scholar]
|
| 5. |
Antic M, Lautenschlager S, Itin PH. Eosinophilic fasciitis 30 years after-what do we really know? Report of 11 patients and review of the literature. Dermatology 2006;213:93-101.
[Google Scholar]
|
| 6. |
Lebeaux D, Sène D. Eosinophilic fasciitis (Shulman disease). Best Pract Res Clin Rheumatol 2012;26:449-58.
[Google Scholar]
|
| 7. |
Chun JH, Lee KH, Sung MS, Park CJ. Two cases of eosinophilic fasciitis. Ann Dermatol 2011;23:81-4.
[Google Scholar]
|
| 8. |
Vasani RJ, Medhekar SV. Generalized morphea developing in a patient previously affected with eosinophilic fasciitis. Indian J Dermatol Venereol Leprol 2012;78:654-6.
[Google Scholar]
|
| 9. |
Heidary N, Cheung W, Wang N, Kamino H, Franks AG Jr. Eosinophilic fasciitis/generalized morphea overlap. Dermatol Online J 2009;15:2.
[Google Scholar]
|
| 10. |
Kreuter A, Kryvosheyeva Y, Terras S, Moritz R, Möllenhoff K, Altmeyer P, et al. Association of autoimmune diseases with lichen sclerosus in 532 male and female patients. Acta Derm Venereol 2013;93:238-41.
[Google Scholar]
|
| 11. |
Kreuter A, Wischnewski J, Terras S, Altmeyer P, Stücker M, Gambichler T. Coexistence of lichen sclerosus and morphea: A retrospective analysis of 472 patients with localized scleroderma from a German tertiary referral center. J Am Acad Dermatol 2012;67:1157-62.
[Google Scholar]
|
| 12. |
Ortega-Loayza AG, Merritt BG, Groben PA, Morrell DS. Eosinophilic fasciitis in a female child. J Am Acad Dermatol 2008;58 5 Suppl 1:S72-4.
[Google Scholar]
|
| 13. |
Endo Y, Tamura A, Matsushima Y, Iwasaki T, Hasegawa M, Nagai Y, et al. Eosinophilic fasciitis: Report of two cases and a systematic review of the literature dealing with clinical variables that predict outcome. Clin Rheumatol 2007;26:1445-51.
[Google Scholar]
|
| 14. |
Frayha RA, Atiyah F, Karam P, Ali Ahmed Z, Salman SM. Eosinophilic fasciitis terminating as progressive systemic sclerosis in a child. Dermatologica 1985;171:291-4.
[Google Scholar]
|
| 15. |
Abbas O, Bhawan J. Sclerosing disorders of the skin: An overview with focus on histopathological features. Am J Dermatopathol 2014;36:763-80.
[Google Scholar]
|
| 16. |
Weedon D, editor. Disorders of collagen. In: Weedon's Skin Pathology. 3rd ed. China: Elsevier; 2010. p. 303-29.
[Google Scholar]
|
| 17. |
Naschitz JE, Boss JH, Misselevich I, Yeshurun D, Rosner I. The fasciitis-panniculitis syndromes. Clinical and pathologic features. Medicine (Baltimore) 1996;75:6-16.
[Google Scholar]
|
| 18. |
Moulton SJ, Kransdorf MJ, Ginsburg WW, Abril A, Persellin S. Eosinophilic fasciitis: Spectrum of MRI findings. AJR Am J Roentgenol 2005;184:975-8.
[Google Scholar]
|
| 19. |
Baumann F, Brühlmann P, Andreisek G, Michel BA, Marincek B, Weishaupt D. MRI for diagnosis and monitoring of patients with eosinophilic fasciitis. AJR Am J Roentgenol 2005;184:169-74.
[Google Scholar]
|
| 20. |
Kirchgesner T, Dallaudière B, Omoumi P, Malghem J, Vande Berg B, Lecouvet F, et al. Eosinophilic fasciitis: typical abnormalities, variants and differential diagnosis of fasciae abnormalities using MR imaging. Diagn Interv Imaging 2015;96:341-8.
[Google Scholar]
|
Fulltext Views
6,646
PDF downloads
2,704





