Translate this page into:
Autoantibody detection by conventional and novel mosaic BIOCHIP technique: A comparative analysis for the diagnosis of autoimmune bullous diseases
Corresponding author: Dr. Raghavendra Rao, Department of Dermatology, Kasturba Medical College, Manipal, Manipal Academy of Higher Education, Manipa, India. raghavrao1@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Suvarna P, Rao R. Autoantibody detection by conventional and novel BIOCHIP mosaic technique: A comparative analysis for the diagnosis of autoimmune bullous diseases. Indian J Dermatol Venereol Leprol. doi: 10.25259/IJDVL_1089_2024
Abstract
Background
Circulating autoantibodies in patients with autoimmune bullous diseases can be detected by indirect immunofluorescence (IIF) microscopy. The sensitivity of this method depends on the substrate used. Normal human skin (NHS) and salt-split skin (SSS) are widely used in conventional serodiagnosis of autoimmune bullous diseases. A novel mosaic biochip has been reported to be a highly sensitive and specific test to detect the circulating antibodies in this subset of patients.
Objectives
This study was designed to compare IIF microscopy by conventional and mosaic biochip technologies in the serodiagnosis of patients with autoimmune bullous diseases.
Methods
This cross-sectional study included sera of 103 patients with autoimmune bullous diseases. Conventional IIF microscopy was carried out using NHS and SSS substrates. IIF using the mosaic biochip was performed as per the manufacturer’s instructions.
Results
The conventional technique detected intercellular staining with IgG in 62 patients, and the mosaic biochip detected it in 56 monkey oesophageal samples. The latter also detected both desmoglein (Dsg) 1 and 3 in 45 patients and Dsg 1 and 3 individually in eight and ten serum samples, respectively. Both techniques detected epidermal staining in the SSS of 37 patients with sub-epidermal autoimmune bullous diseases, and dermal staining was observed in seven sera. Seventeen patients with epidermal staining patterns revealed antibodies to BP180 only; six patients showed reactivity to both BP 180 and 230.
Limitation
Due to financial constraints, sera of healthy controls could not be studied.
Conclusions
The BIOCHIP mosaic IIF is an useful adjunct IIF technique. It not only helps to detect the staining pattern but also identify the target antigens in common autoimmune bullous disease. Sensitivity of conventional IIF in detecting ICS is 93.9% in contrast to 84.4% with the BIOCHIP mosaic technique. However, mosaic BIOCHIP technique helped to identify the target antigens in common AIBD.
Keywords
Autoimmune bullous diseases
bullous pemphigoid
indirect immunofluorescence
mosaic biochip
pemphigus
Introduction
The diagnosis of autoimmune blistering diseases (AIBDs) involves a multi-step approach combining clinical examination, histopathology, direct immunofluorescence (DIF), Indirect immunofluorescence (IIF), and enzyme-linked immunosorbent assay (ELISA). DIF microscopy is the gold standard test for the diagnosis of AIBDs. Circulating autoantibodies can be assessed by IIF microscopy. DIF and IIF, however, give very little information about the target antigens.1,2 ELISA or immunoblotting helps to detect the target antigens. As a quantitative test, ELISA also detects the antibody burden in the body. These tests are time-consuming, require different substrates, and demand specific testing protocols. BIOCHIP (Dermatology Mosaic 7, Euroimmun, Lübeck) mosaic-based IIF technique has been developed as a novel single-step approach to diagnose AIBDs. The BIOCHIP is designed to enable the visualisation of multiple antigenic structures in separate windows on a single incubation field.3 In this study, we compared conventional IIF to BIOCHIP mosaic-based IIF.
Methods
A cross-sectional study was conducted among patients with a clinical diagnosis of AIBD attending the dermatology department of a tertiary care Kasturba hospital, Manipal between August 2020 and December 2021. Patients were included in the study only if the AIBD diagnosis was confirmed by DIF microscopy. Sera of 103 patients were collected irrespective of the disease activity (sample size was calculated using 4pq/d2; p=80%, q=p-1, d=10% of p). Sera of patients who revealed exclusive IgA deposition under DIF were excluded. IIF was performed by the conventional method and then using BIOCHIP mosaic slides, as described below. Written informed consent was obtained from all participants; the study was approved by the Institutional Ethics Committee (IEC 780/2019) and registered under the Clinical Trials Registry of India (CTRI/2020/01/022752).
The conventional two-step IIF procedure was carried out on frozen sections of normal human skin (NHS) and salt-split skin (SSS) as substrates. SSS were prepared by overnight incubation of NHS in one molar sodium chloride (NaCl) at room temperature. NHS were gently teased with a blunt forceps to separate the epidermis from the dermis. Subsequently, 6 µm frozen sections of NHS and SSS were obtained and incubated in the patients’ sera (1:10 dilution) for one hour in a moist chamber. The sections were then washed and stained with fluorescein-labelled anti-human IgG antibodies for one hour in a moist chamber.
IIF using BIOCHIP mosaic technique was performed using Dermatology Mosaic 7 slides. Each slide had five incubation fields, and each incubation field had six windows, as shown in Figure 1. IIF was carried out as per the manufacturer’s instructions.
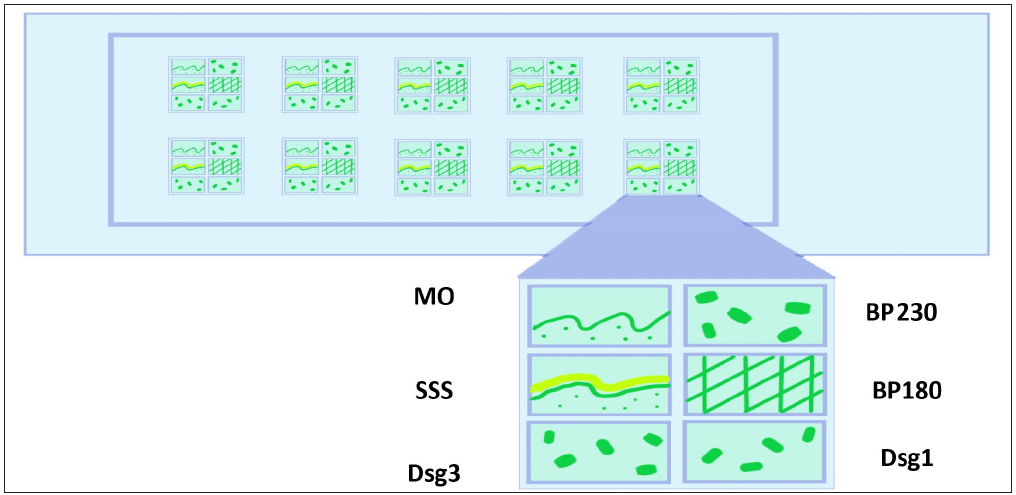
- Schematic diagram showing various substrates in mosaic biochip 7 slide Each window of the BIOCHIP slide was pre-coated with six different substrates: MO, normal human salt split skin, human embryonic kidney (HEK293) cells transfected with desmoglein (Dsg) 1 protein ectodomain, HEK293 cells transfected with Dsg 3 protein ectodomain, micro drops of non-collagenous 16 A domain of BP180 free antigen (BP180NC16A), and HEK293 cells transfected with C-terminal globular domain of the BP230 domain. (MO: Monkey oesophagus, SSS: Salt split skin, Dsg3: Desmoglein 3, Dsg1: Desmoglein1, BP 180: Bullous pemphigoid 180, BP 230: Bullous pemphigoid 230.)
Results
There were 55 males and 48 females in the study group, and the average age of patients was 51.7±17.2 years (range 12-86 years). The most common clinical diagnosis was pemphigus vulgaris (PV) (52 patients; 50.5%), followed by bullous pemphigoid (BP) (28 patients; 27.2%). Other clinical diagnoses included pemphigus foliaceus (PF) (12 patients; 11.7%) and epidermolysis bullosa acquisita (EBA) (7 patients; 6.8%). A differential diagnosis of various AIBDs was considered in four patients. The mean duration of the disease was 17.8 months. Based on DIF microscopy, a revised diagnosis of pemphigus was considered in 66 patients (64.1%) including 54 (81.8%) with PV and 12 (18.2%) with PF. Sub-epidermal AIBD diagnosis was confirmed in 37 patients by linear staining of the basement membrane zone (BMZ) with IgG and C3.
Conventional IIF using NHS substrates revealed intercellular staining (ICS) with IgG in 62 patients (93.9%) with pemphigus. The monkey oesophagus (MO) sections in the mosaic biochip technique detected ICS in 56 substrates (84.4%) [Table 1]. By the mosaic technique, 45 serum samples (68.2%) showed reactivity against both desmoglein (Dsg) 1 and 3, while it was individually positive for Dsg1 and Dsg3 in eight (12.2%) and ten serum samples (15.2%), respectively [Figure 2]. Interestingly, seven sera from pemphigus patients, which did not reveal ICS with MO, showed reactivity with Dsg1 and 3. Of the 12 patients with PF, sera of five (41.7%) reacted exclusively with Dsg1, while seven (58.3%) showed reactivity with both Dsg1 and Dsg3. Among 54 patients with PV, 10 (18.5%) showed exclusive reactivity with Dsg3; six among them had mucocutaneous lesions, whereas four had only mucosal lesions. Three patients (5.5%) with PV showed exclusive reactivity to Dsg1 despite having mucosal lesions.
| Intraepidermal AIBD | |||||||
|---|---|---|---|---|---|---|---|
| Conventional IIF using NHS | Biochip mosaic | ||||||
| ICS +’ve (n;%) | ICS -’ve (n;%) | ICS+ve MO (n;%) | ICS-ve MO (n;%) | Both Dsg 1& 3 +’ve (n;%) | Only Dsg1 +’ve (n;%) | Only Dsg3+’ve (n:%) | |
| PV (n=54) | 52 (96.3) | 02 (3.7) | 46 (85.2) | 08 (14.8) | 38 (70.4) | 03 (5.5) | 10 (18.5) |
| PF (n=12) | 10 (83.3) | 02 (16.7) | 10 (83.3) | 02 (16.7) | 07 (58.3) | 05 (41.7) | Nil |
| Total (n=66) | 62 (93.9) | 04 (6.06) | 56 (84.4) | 10 (15.2) | 45 (68.2) | 08 (12.2) | 10 (15.2) |
IIF: Indirect immunofluorescence, AIBD: Autoimmune bullous disease, MO: Monkey oesophagus, ICS: Intercellular staining, PV: Pemphigus vulgaris, PF: Pemphigus foliaceus
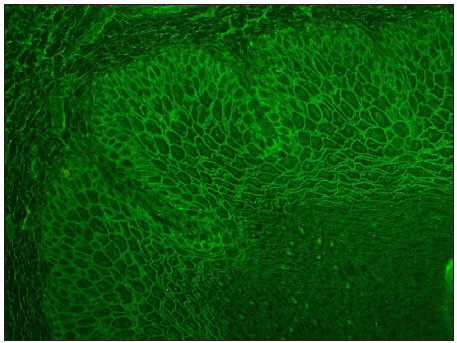
- Photomicrograph showing intercellular staining of MO with IgG in 1:10 dilution (FITC, 200x). (FITC: Fluorescein isothiocyanate conjugate)
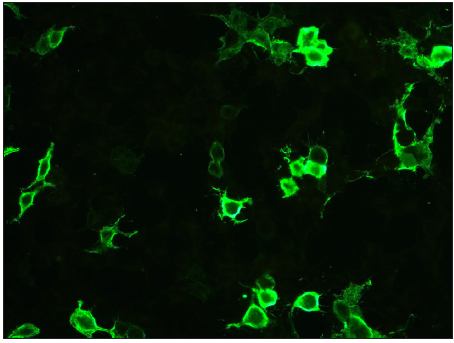
- Photomicrograph showing positive staining on Dsg1 substrate (FITC, 200x).
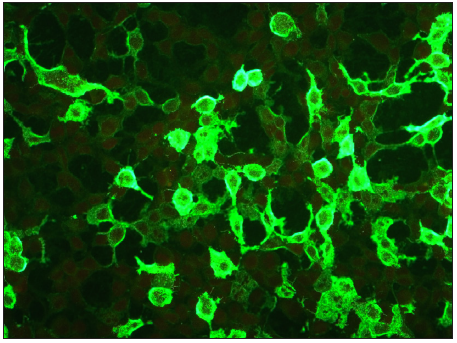
- Photomicrograph showing positive staining on Dsg3 substrate in PV (FITC, 200x).
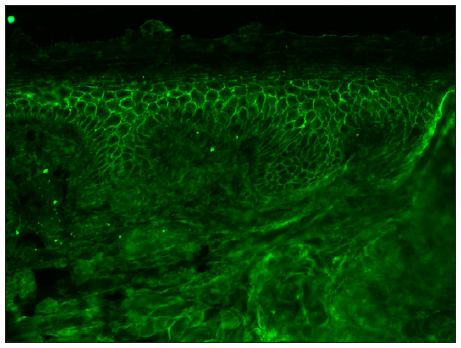
- Photomicrograph showing intercellular staining of the epidermis with IgG in NHS by conventional technique in pemphigus (FITC, 200x).
Among patients with AIBD (n=37), 29 (78.4%) sera were detected with epidermal staining in SSS, and seven (18.9%) showed dermal staining. In one patient’s serum, neither IIF technique could detect circulating BMZ antibodies. The majority of patients with epidermal staining patterns revealed antibodies only against BP180 (n=17; 58.6%); six patients (20.7%) showed reactivity to both BP180 and BP230 [Figure 3]. Exclusive BP230 reactivity was detected in only one serum (3.4%). In seven patients showing a dermal staining patterns, circulating autoantibodies against BP180 and BP230 could not be detected by IIF.
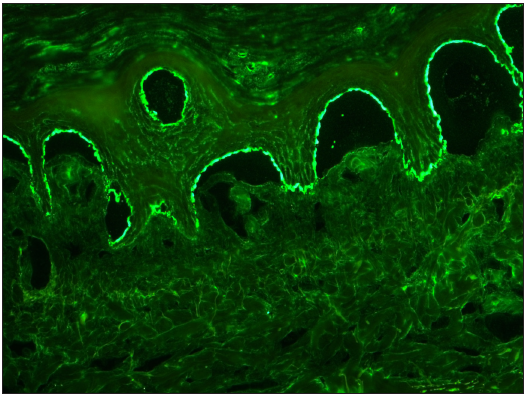
- Photomicrograph showing epidermal staining (‘roof” pattern) in SSS with IgG in 1:10 dilution (FITC, 200x).

- Photomicrograph showing positive staining of BP180 substrate (FITC, 200x).
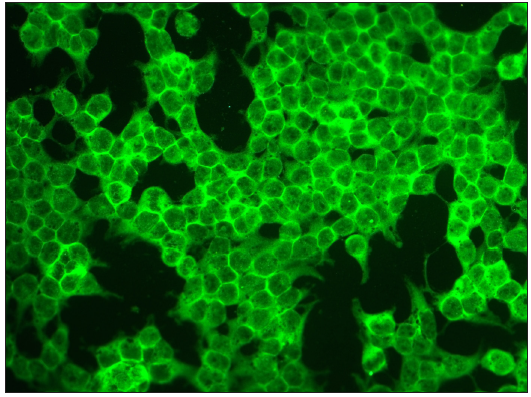
- Photomicrograph showing positive staining BP230 substrates in BP (FITC, 200x).
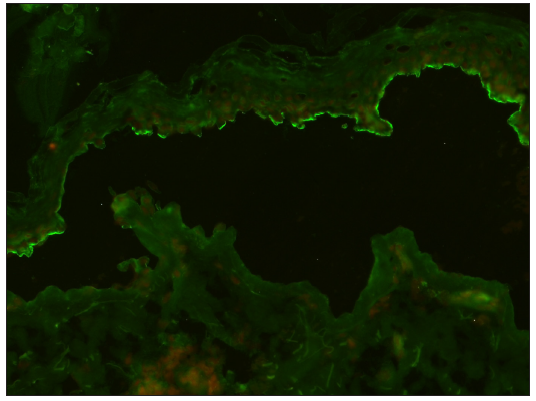
- Photomicrograph showing linear staining of BMZ on the epidermal side (‘roof” pattern) of the split in the SSS in BP using conventional technique (FITC, 200x).
Discussion
Serological diagnosis plays a crucial role in the management of AIBDs as it not only helps in the sub-classification of the clinical condition but also has prognostic implications. Detection of serum autoantibodies is a multi-step procedure comprising an initial screening step by IIF microscopy followed by more specific tests that aim at identifying the target antigen(s).4,5 An ideal test should help the clinician swiftly diagnose the most common AIBDs so that immediate and often aggressive treatment can be instituted.6,7
In 2012, van Beek and colleagues described a novel IIF-based diagnostic technique using BIOCHIP mosaic-7 slides. Each slide has 5-10 incubation fields; each incubation field has six windows coated with different antigenic structures, enabling the diagnosis and sub-categorisation of common AIBDs within an hour using a small quantity of serum. They showed that the diagnostic efficacy of the BIOCHIP mosaic 7 was comparable to the conventional multi-step procedure in the diagnosis of AIBD.3 Using the BIOCHIP mosaic, sensitivity of the Dsg1, Dsg3, and BP180 NC16A-specific substrates were 90%, 98.5%, and 100%, respectively. BP230 was recognised by 54% of the BP sera. Specificity ranged between 98.2% and 100% for all substrates.3
ICS using NHS substrates was detected in 93.9% of patients with conventional IIF and in 85.85% of patients using the BIOCHIP mosaic [Table 2]. Anti-Dsg3 and anti-Dsg1 antibodies could be demonstrated in 83.3% and 80.3% of patients, respectively. Subcategory analysis of pemphigus patients revealed anti-Dsg3 (88.9%) to be more common than anti-Dsg1 (75.9%) in PV patients. Conversely, anti-Dsg1 (100%) was detected more frequently than anti-Dsg3 (58.3%) among PF patients. In PV, the sensitivity of anti-Dsg3 on the BIOCHIP has been reported to range from 60.9% to 100%.8-10 The sensitivity of anti-Dsg1 is reported to be much lower, between 13% and 52.3%.8-12 This reflects the nature of PV, where autoantibodies against anti-Dsg1 are only expected in mucocutaneous forms of the disease. For PF, the sensitivity of anti-Dsg1 transfected cells on the BIOCHIP has been reported to be between 75% and 90%. Tampoia et al. reported ICS, anti-Dsg3, and anti-Dsg1 in 83.3%, 100%, and 33.3% of patients, respectively, in 36 PV cases.8 Ozkesici et al., in their study of 45 pemphigus patients, detected ICS staining of the MO sections in 31 patients (68.9%), anti-Dsg3 in 39 (86.7%) and anti-Dsg1 in 17 (37.8%).9 In a study by Tirumale et al., anti-Dsg3 was the most common positive substrate in the pemphigus group (58/67, 86%), followed by anti-Dsg1 (73%). However, their study detected ICS only in 33/67 patients (49%).11 Russo et al. studied 42 patients with PV and detected anti-Dsg3 antibodies in 41 (97.6%) and anti-Dsg1 antibodies in 19% of patients.12
| PV | PF | |||||
|---|---|---|---|---|---|---|
| MO (n;%) | Dsg1 (n;%) | Dsg3 (n;%) | MO (n;%) | Dsg1 (n;%) | Dsg3 (n;%) | |
| van Beek et al. (2012)3 |
65/65 (100) |
33/65 (50.8) | 64/65 (98.5) |
49/50 (98) |
45/50 (90) |
3/50 (6) |
| Tampoia et al. (2012)8 | 30/36 (83.3) | 12/36 (33.3) |
36/36 (100) |
- | - | - |
| Arunaprasath et al. (2020)10 |
18/18 (100) |
15/18 (83.3) |
16/18 (88.8) | - | - | - |
| Yang et al. (2019)14 |
14/23 (60.9) |
3/23 (13) |
14/23 (60.9) | 7/8 (87.5) | 6/8 (75) | 5/8 (62.5) |
| Current study | 46/54 (85.2) | 41/54 (75.9) | 48/54 (88.8) | 10/12 (83.3) | 12/12 (100) | 07/12 (58.3) |
MO: Monkey oesophagus
Both methods detected Anti-BMZ antibodies using SSS in 36/37 patients (97.3%) with sub-epidermal AIBDs. The epidermal staining pattern was detected in 78.4% of patients, while the dermal staining pattern was seen in 18.9%. Adaszewska et al. studied 51 patients with BP and found that anti-BMZ staining could be detected on the epidermal side of the split by both conventional and mosaic biochip techniques in 48 patients (94.1%).13 Anti-BP180 and anti-BP230 antibodies were detected in 79.3% and 24.1% of patients, respectively, in the present study. Among BP patients, the sensitivity of BP180 mosaic on the BIOCHIP has been found to range between 55.3% and 100%, and a specificity between 96.5% and 100%.8-14 On the other hand, sensitivity (45.1%–66.7%) and specificity (39%–100%) of BP230 on mosaic BIOCHIP is relatively less. The lower sensitivity of the BP230 mosaic is not unexpected, given that it is more common to have autoantibodies against BP180 in BP.8-14 van Beek et al., in their study of 42 BP patients, found BMZ staining in SSS in 41 sera (98.8%), whereas BP180 and BP230 antibodies were detected in 100% and 54.8% of patients, respectively.3 Yang et al found the sensitivity and specificity of the BP180 substrate were 55.3% and 97.7%, respectively, whereas the sensitivity and specificity of the BP230 substrate were 65.8% and 86.5%, respectively.6 However, in other studies, the sensitivity of the BIOCHIP for the detection of BP180 (83.3-100%) and BP230 (24.3-66.7%) were variable8-14 [Table 3].
| Subepidermal AIBD | ||||
|---|---|---|---|---|
| Epidermal binding with IgG on SSS (n;%) | Dermal binding with IgG on SSS (n;%) | BP 180 (n;%) | BP 230 (n;%) | |
| van Beek et al. (2012)3 | 41/42 (97.6) | NA | 42/42 (100%) | 23/42 (54.8) |
| Tampoia et al. (2012)8 | NA | NA | 36/40 (90) | 16/40 (40) |
| Özkesici et al. (2017)9 | 15/18 (83.3) | NA | 16/18 (88.8) | 12/18 (55.5) |
| Arunaprasath et al. (2020)10 | 18/22 (81.8) | 4/22(18.2) | 17/22 (77.3) | 15/22 (68.2) |
| Adaszewska et al. (2020)13 | 48/51 (94.1) | - | 39/51 (76.5) | 23/51(45.1) |
| Yang et al. (2019)14 | 29/38(76.3) | - | 21/38 (55.3) | 25/38(65.8) |
| Current study | 29/37 (78.4) | 7/37 (18.9) | 23/29 (79.3) | 7/29 (24.1) |
NA: Not available
Six serum samples with ‘dermal binding” on SSS did not react with either BP180 or BP230. BIOCHIP 7 lacked antigens, which are the target in dermal binding subepidermal AIBD. Euroimmun has recently introduced an extended series with 12 miniature biochips, which enables the diagnosis of EBA, paraneoplastic pemphigus (PNP), and dermatitis herpetiformis (DH) but not anti-laminin 332 mucous membrane pemphigoid and anti-p200 pemphigoid.15
Limitations
Limitations of the study include lack of a control group of healthy individuals and not performing ELISA simultaneously; it would have helped us to compare the antibody profile by two techniques.
Conclusion
The BIOCHIP mosaic is a rapid test based on IIF microscopy that can be performed to screen circulating antibodies in suspected AIBD patients. In the present study, the sensitivity of conventional IIF in detecting ICS is 93.9% in contrast to 84.4% with the BIOCHIP mosaic technique. In addition to identifying staining patterns in the tissue sections, the mosaic technique helped to identify antigen targets in common AIBD. However, it is more expensive than conventional IIF and semiquantitative in nature, necessitating the need to perform ELISA to quantify antibody burden among patients. Nevertheless, BIOCHIP mosaic IIF offers value addition in diagnosing AIBD.
Acknowledgment
Authors thank Dr SL Sujitha Reddy, Junior resident, Department of Dermatology, Kasturba Medical College, Manipal for drawing Figure 1.
Ethical approval
The research/study was approved by the Institutional Review Board at Kasturba Medical College, Manipal, number IEC 780/2019, dated 13/9/2019.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Financial support and sponsorship
Authors have received IADVL research grant 2019 for this study.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
References
- Diagnosis of autoimmune blistering diseases. Front Med (Lausanne). 2018;5:296.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Diagnosis of autoimmune bullous diseases. J Dtsch Dermatol Ges. 2018;16:1077-91.
- [CrossRef] [Google Scholar]
- Serological diagnosis of autoimmune bullous skin diseases: Prospective comparison of the BIOCHIP mosaic-based indirect immunofluorescence technique with the conventional multi-step single test strategy. Orphanet J Rare Dis. 2012;7:49.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Importance of serological tests in diagnosis of autoimmune blistering diseases. J Dermatol. 2015;42:3-10.
- [CrossRef] [PubMed] [Google Scholar]
- S2k guideline for the diagnosis of pemphigus vulgaris/foliaceus and bullous pemphigoid. J Dtsch Dermatol Ges. 2015;13:713-27.
- [CrossRef] [PubMed] [Google Scholar]
- Validation of the BIOCHIP test for the diagnosis of bullous pemphigoid, pemphigus vulgaris and pemphigus foliaceus. J Eur Acad Dermatol Venereol. 2020;34:153-60.
- [CrossRef] [PubMed] [Google Scholar]
- Serological diagnosis of autoimmune bullous skin diseases. Front Immunol. 2019;10:1974.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Anti-skin specific autoantibodies detected by a new immunofluorescence multiplex biochip method in patients with autoimmune bullous diseases. Dermatology. 2012;225:37-44.
- [CrossRef] [PubMed] [Google Scholar]
- The value of the BIOCHIP mosaic-based indirect immunofluorescence technique in the diagnosis of pemphigus and bullous pemphigoid in turkish patients. Acta Dermatovenerol Croat. 2017;25:202-9.
- [PubMed] [Google Scholar]
- Comparative analysis of BIOCHIP mosaic-Based indirect immunofluorescence with direct immunofluorescence in diagnosis of autoimmune bullous diseases: A cross-Sectional study. Indian Dermatol Online J. 2020;11:915-9.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Role of BIOCHIP indirect immunofluorescence test in cutaneous vesiculobullous diseases. Am J Dermatopathol. 2020;42:322-8.
- [CrossRef] [PubMed] [Google Scholar]
- The use of biochip immunofluorescence microscopy for the diagnosis of pemphigus vulgaris. Acta Histochem. 2014;116:713-6.
- [CrossRef] [PubMed] [Google Scholar]
- The use of BIOCHIP mosaics in diagnostics of bullous pemphigoid: Evaluation and comparison to conventional multistep procedures. J Cutan Pathol. 2020;47:121-7.
- [CrossRef] [PubMed] [Google Scholar]
- A new indirect immunofluorescence BIOCHIP method for the serological diagnosis of bullous pemphigoid: A review of literature. Australas J Dermatol. 2019;60:e173-7.
- [CrossRef] [PubMed] [Google Scholar]
- Multicenter prospective study on multivariant diagnostics of autoimmune bullous dermatoses using the BIOCHIP technology. J Am Acad Dermatol. 2020;83:1315-22.
- [CrossRef] [PubMed] [Google Scholar]







