Translate this page into:
Autologous fibroblast suspension for the treatment of refractory diabetic foot ulcer
2 Skin and Stem Cell Research Center, Tehran University of Medical Sciences, Tehran; Skin Diseases and Leishmaniasis Research Center, Isfahan University of Medical Sciences, Isfahan, Iran
3 Isfahan Endocrine and Metabolism Research Center, Isfahan University of Medical Sciences, Isfahan, Iran
4 Skin Diseases and Leishmaniasis Research Center, Isfahan University of Medical Sciences, Isfahan, Iran
5 Department of Dermatology, Isfahan University of Medical Sciences, Isfahan, Iran
Correspondence Address:
Amir Hossein Siadat
Skin Diseases and Leishmaniasis Research Center, Department of Dermatology, Isfahan University of Medical Sciences, Isfahan
Iran
| How to cite this article: Nilforoushzadeh MA, Jaffary F, Siavash M, Ansari N, Siadat AH, Heidari A. Autologous fibroblast suspension for the treatment of refractory diabetic foot ulcer. Indian J Dermatol Venereol Leprol 2016;82:105-106 |
Ulcers of the lower extremities, usually caused by vascular insufficiency or peripheral neuropathy, are regarded as serious complications of diabetes mellitus.[1] At least 15% of diabetic patients may be affected by diabetic foot ulcer over their lifetime.[1] One recent case series showed that in vitro expanded fibroblasts were a satisfactory therapeutic option to treat large leg ulcers in patients with diabetes.[2]
Our patient was a 62-year-old man who had diabetes mellitus for 30 years. Following contact with a hot metal, he developed a large ulcer of dimensions 9.93 × 9.09 cm with an area of 67.960 cm 2 and a depth of 4 mm (stage B, grade 2) on the medial surface of his right foot. Diameters, area and depth of the ulcer were determined by Pictzar software before and 4 months after treatment. Despite classic diabetic wound care for the patient which included off-loading, surgical debridement, topical and oral antibiotic treatment (including ciprofloxacin 500 mg, twice daily and clindamycin 300 mg, thrice daily) and glycemic control, no sign of improvement was observed after 6 weeks [Figure - 1]. The stage and grade of the wound was defined according to University of Texas wound classification system.[3] The wound infection score was based on the system from Knighton et al., as modified by Pecoraro et al.[4]
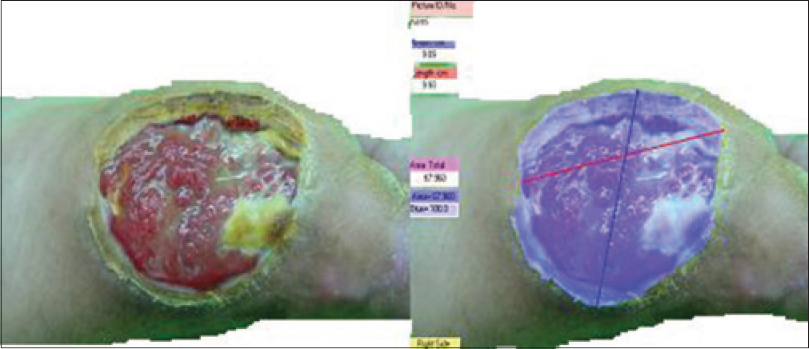 |
| Figure 1: The ulcer before tricloroacetic acid and fibroblast application |
The hyperkeratotic surrounding tissue was chemically debrided with three sessions of 50% trichloroacetic acid (TCA) application. The ulcer size reduced to a diameter of 5.11 × 4.73 cm with an area of 13.880 cm 2, and a depth of 2 mm (stage B, grade 1) [Figure - 2]. For obtaining autologous cultured fibroblasts, a 4 mm 2 punch biopsy was taken from the retro-auricular region after regional anesthesia with lidocaine, 2%. In addition, 50 cc blood was taken from the patient and both the skin sample and blood were transferred to the Rooyan institute for fibroblast culture.
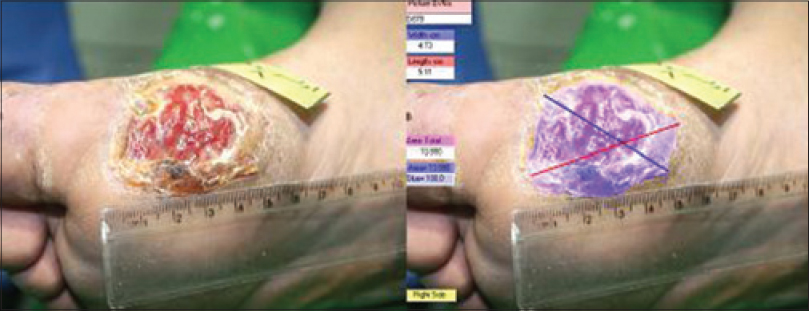 |
| Figure 2: The ulcer after trichloroacetic acid debridement and before fibroblast application |
At the Rooyan institute, the fatty layer of the skin sample was removed and the remaining skin was minced into small pieces and placed in culture flasks containing Dulbecco's Modified Eagle Medium (DMEM) F12+ with 100 µg/ml streptomycin, 10% autologous human serum, 100 IU/ml penicillin and maintained at 37°C and 5% CO2 and 95% humidity. After 3 weeks, 20 million fibroblasts were isolated and were suspended in phosphate-buffered saline (PBS) for transplantation.
To prepare the wound for cell transplantation, in addition to surrounding hyperkeratotic tissue, the base of the ulcer was abraded using a surgical scalpel blade No. 15 until pinpoint bleeding occurred. A thin layer of fibroblast suspension was applied to the ulcer base using a sterile syringe and the ulcer surface was covered with sterile vaseline gauze and 3 M Tegaderm (which consists of a thin polyurethane membrane coated with a layer of an acrylic adhesive) and a crepe bandage. Cephalexin, 500 mg four times daily was prescribed for 7 days to prevent secondary infection. The dressing was removed on day 7.
The ulcer showed significant granulation tissue at this time. Two weeks after fibroblast application, the patient showed the first signs of re-epithelialization; the depth and diameter of the ulcer was reduced and it was completely re-epithelialized within 4 months (wound infection score: 0). The residual scar had a diameter of 2.25 × 2.59 cm, area of 2.427 cm 2, and depth of 0 mm [Figure - 3]. A small biopsy taken from the treated site to check for malignant change showed normal scar tissue.
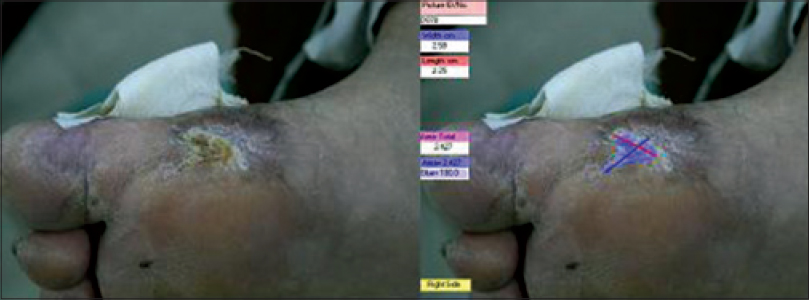 |
| Figure 3: The ulcer after 4 months fibroblast application |
Fibroblasts are mesenchymal cells that play an important role in epithelial-mesenchymal interactions. They secrete various growth factors and cytokines that have a direct effect on epidermal proliferation, differentiation and formation of extracellular matrix. Fibroblasts have been incorporated into various tissue-engineered products such as Dermagraft (Advanced BioHealing, La Jolla, CA, USA) and Apligraf (Novartis, Basel, Switzerland) and have a variety of clinical applications in dermatology and plastic surgery including treatment of burns and chronic venous ulcers.[5] Cultured fibroblast suspension appeared to significantly enhance wound healing in our patient.
Fourteen days after fibroblast suspension transfer, the ulcer was completely filled with granulation tissue and showed signs of re-epithelization and at 4 months the ulcer was completely healed. Dressing was continued using regular sterile gauze dressing. The patient continued his normal activity during this period. This accelerated healing might have contributed to reducing complications such as infection, achieving a better outcome and also to potentially reducing the total cost of treatment for the patient.
In conclusion, the good response of this non-healing ulcer to trichloroacetic acid debridement and cultured fibroblast suspension along with the results of a previous study,[5] may suggest that the application of cultured fibroblasst is an effective method for the treatment of some selected refractory diabetic ulcers.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Smith J. Debridement of diabetic foot ulcers. Cochrane Database Syst Rev 2002:CD003556.
[Google Scholar]
|
| 2. |
Cavallini M. Autologous fibroblasts to treat deep and complicated leg ulcers in diabetic patients. Wound Repair Regen 2007;15:35-8.
[Google Scholar]
|
| 3. |
Lavery LA, Armstrong DG, Harkless LB. Classification of diabetic foot wounds. J Foot Ankle Surg 1996;35:528-31.
[Google Scholar]
|
| 4. |
Lipsky BA, Holroyd KJ, Zasloff M. Topical versus systemic antimicrobial therapy for treating mildly infected diabetic foot ulcers: A randomized, controlled, double-blinded, multicenter trial of pexiganan cream. Clin Infect Dis 2008;47:1537-45.
[Google Scholar]
|
| 5. |
Nilforoushzadeh MA, Nasr Esfahani MH, Fesharaki MA, Siadat AH, HaftBaradaran E. Treatment of Recalcitrant Electrical Burn Ulcer with Application of Topical Trichloroacetic Acid and Autologous Cultured Fibroblast. Cell Tissue Transplant Ther 2010;3:1-4.
[Google Scholar]
|
Fulltext Views
3,616
PDF downloads
2,416





