Translate this page into:
Bacterial contamination of the hands of doctors: A study in the medicine and dermatology wards
2 Department of Dermatology, Medical College Kolkata, Calcutta, India
3 Department of Microbiology, NRS Medical College, Calcutta, India
Correspondence Address:
Rudrajit Paul
15/5 Bose Pukur Road, Calcutta - 700 039
India
| How to cite this article: Paul R, Das NK, Dutta R, Bandyopadhyay R, Banerjee AK. Bacterial contamination of the hands of doctors: A study in the medicine and dermatology wards. Indian J Dermatol Venereol Leprol 2011;77:307-313 |
Abstract
Background: Doctors' hands are a common source of bacterial contamination. Often, these organisms are found to be virulent species with multidrug-resistance patterns. These are the sources of nosocomial infections in many patients. Aims: The present study was undertaken to find out the prevalence of bacterial contamination in the hands of doctors in the Medicine and Dermatology wards of a tertiary care hospital. Methods: The hands of 44 doctors were swabbed and cultured at entry to ward and at exit. Then, tap water and alcohol swab wash techniques were used and further swabs were done at each step. Thus, each doctor was sampled four-times for the study. The antibiotic-sensitivity pattern of the organisms was determined by the disc-diffusion method. Results: There was a significant contamination of the doctors' hands at entry (59.1%) and at exit (90.9%). Overall, Staphylococcus was the predominant organism (59% at entry and 85% at exit); coagulase-negative ones were more prevalent at entry (32%) and coagulase-positive ones were more prevalent at exit (54%). There was no difference in the hand contamination rates of junior and senior doctors. Also, the contamination rates were similar in the Medicine and Dermatology wards. Among the Gram negative organisms, Escherichia coli (4.5%), Pseudomonas (4.5%), Enterococci (13.6%) and Klebsiella (9%) were the main ones isolated. Gram negative organisms were significantly more prevalent at exit (P = 0.009) compared with their numbers at entry. Hand washing techniques reduced the contamination rates significantly, 76% with tap water wash and further 16.5% with alcohol swab. The removal rate for both groups of organisms was similar. Also, coagulase-positive and -negative Staphylococci showed equal rates of removal with hand washing (P = 0.9793). The organisms were found to be resistant to most of the commonly used antibiotics; the beta-lactam group was especially largely resistant both for Gram positive and Gram negative bacteria. Both cheaper ones like cloxacillin (50-100%) and very costly ones like cefepime (100%) were equally vulnerable to resistance. Even newer antibiotics like linezolid and vancomycin showed a significant resistance to Staphylococcus. In Gram negative organisms, drugs like ceftazidime and gentamicin showed 100% resistance. Conclusion: This study shows the high level of contamination of doctors' hands. It emphasizes the need for proper hygienic measures in day to day practice in hospitals to reduce the level of nosocomial infections. Also, it shows that most of the commonly used antibiotics will be ineffective in nosocomial infections.Introduction
Nosocomial infections are an important cause of mortality and morbidity in any hospital setting. [1] Of them, infections contracted from doctors occupy a sizeable proportion. Person-to-person contact among medical staff and between medical staff and patients appears to be the most common route of transmission of virulent strains like Staphylococcus aureus (including methicillin-resistant Staphylococcus aureus, i.e. MRSA) and Pseudomonas.[2] The hands and stethoscopes of the doctors are long known as sources of bacterial transfer. The present study aims at finding the prevalence of pathogenic bacteria in the hands of doctors in the Departments of Medicine and Dermatology in a tertiary care hospital of Eastern India. This is a pilot study to find out the level of contamination of doctors′ hands, the resistance pattern of the organisms isolated and the effect of hand wash on the decrease in colonization. The objectives of the study were:
- To study the prevalence of bacterial colonization of the hands of doctors and the relative prevalence of the bacteria isolated.
- To determine the antibiotic-sensitivity pattern of the bacteria isolated.
- To find out the effect of hand wash on decrease in colonization.
Methods
The study was conducted from 01 February to 30 November, 2009, in the Medicine and Dermatology wards of the Institution after the study protocol was approved by the institutional ethics committee. A total of four wards consisting of 232 beds were included in the study. A total of 44 doctors of all designations were included in the study, although the major portion was junior doctors (n = 30). The swabs were taken at entry in the ward and also at exit from the ward without hand wash. Then, swabs were taken after hand washing with tap water and, subsequently, after alcohol swabs. Therefore, each doctor was sampled four-times for the study [Figure - 1]. The wards of the hospital are cleaned twice-daily with savlon/phenol solution for the floors. The bed sheets are changed daily and insect repellants are sprayed once-daily. Decontamination of stationary items on a daily basis is not possible. The collection of the samples was performed at random and the participating doctor was not sensitized prior to sample collection. However, when a doctor was involved at entry to ward, he was not told about the subsequent swab tests at exit. The doctors who were tested were requested not to tell anyone of the study. Usually, testing a doctor in front of another was avoided as far as possible. However, in case two or three came together, all were included. This was done to ensure that the participants do not exercise any extra effort in cleaning their hands excepting their routine practice, although informed written consent was obtained prior to sample collection. On the morning of the study, the names of the doctors to be tested were selected at random from the duty roster by any one investigator. No one else was informed of the names. A doctor once selected was excluded subsequently. However, there is always chance of doctors making an extra effort to clean their hands once they were included in the study.
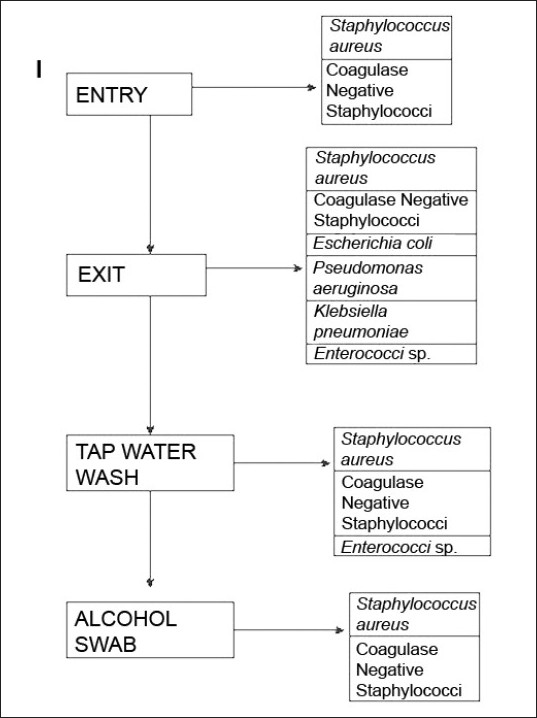 |
| Figure 1: Algorithm depicting the study design and the isolated bacteria at various stages |
The cotton swabs, moistened with sterile physiological saline, were used to collect samples from all the fingers and tip of nails, including finger-rings (if worn by the participants) of both hands. The swabs were immediately (<2 h) streaked onto two sets of three plates that consisted of nutrient agar, blood agar supplemented with 5% defibrinated sheep blood and MacConkey Agar. A Gram stain from the swabs was done prior to plating. In case the organism count was very low (<1/high-power field) the swab was cultured in nutrient broth. Plates were incubated as follows: one set aerobically at 37ºC and the other set anaerobically for 48 h each. In case of nutrient broth, after 48 h of incubation, again plating was carried out as above. Isolated microorganisms were identified using Gram′s stain, hemolysis patterns and colony morphology [Figure - 2] and [Figure - 3]. Gram positive and -negative ones were identified by standard methods. [3] Incubation was done up to 72 h before declaring negative for growth.
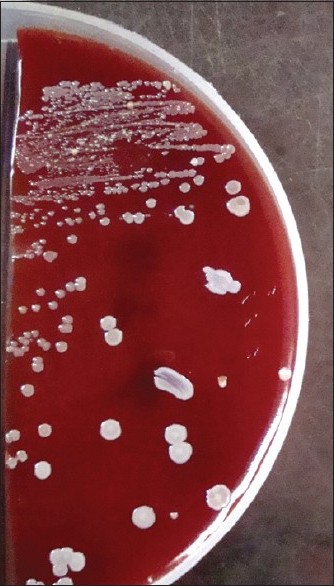 |
| Figure 2: Blood agar showing growth of Staphylococcus aureus |
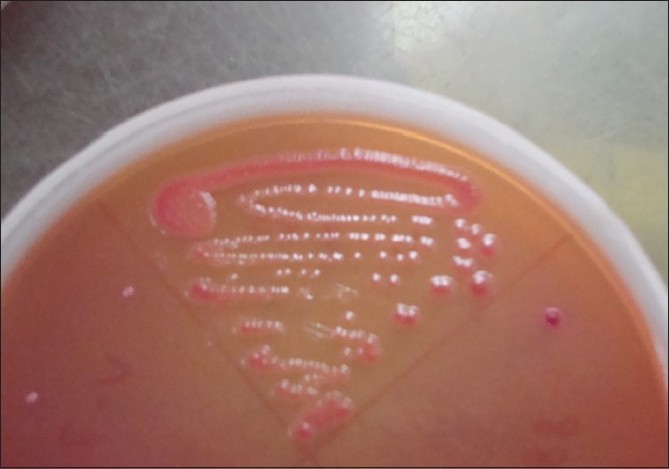 |
| Figure 3: MacConkey agar showing growth of Escherichia coli |
Any growth was analyzed for sensitivity by the disc-diffusion method (Kirby-Bauer method) only [4]
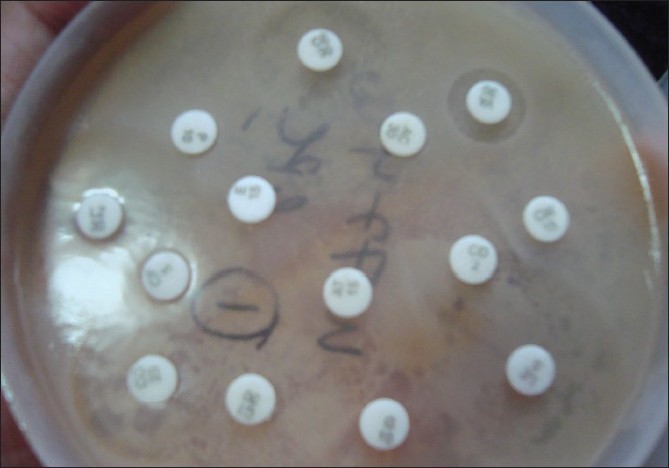 |
| Figure 4: Disc-diffusion method of antibiotic-sensitivity testing |
The data were analyzed using MedCalc statistical software. Categorical variables are presented as simple frequencies and proportions and analyzed by the Chi-square test and Fisher′s exact test, as applicable. P-values <0.05 were considered statistically significant.
Results
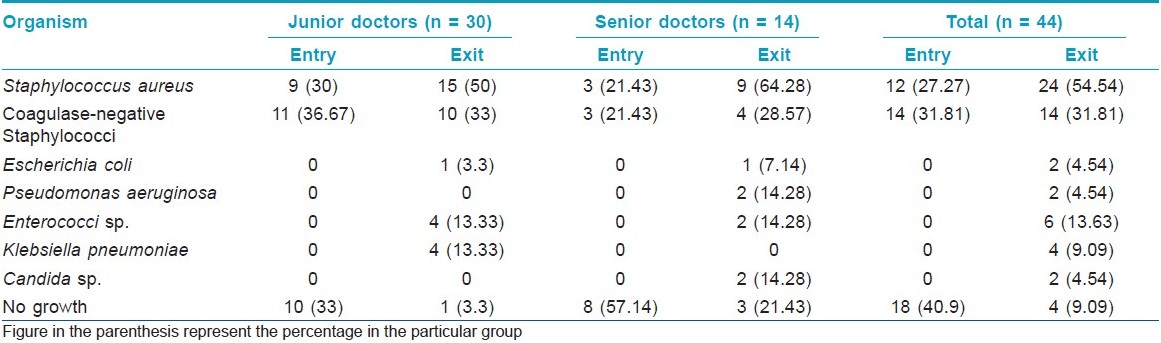
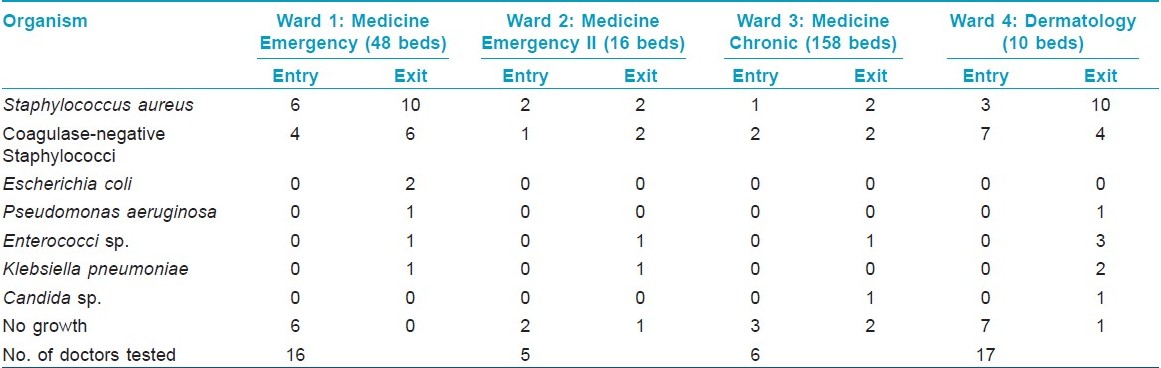
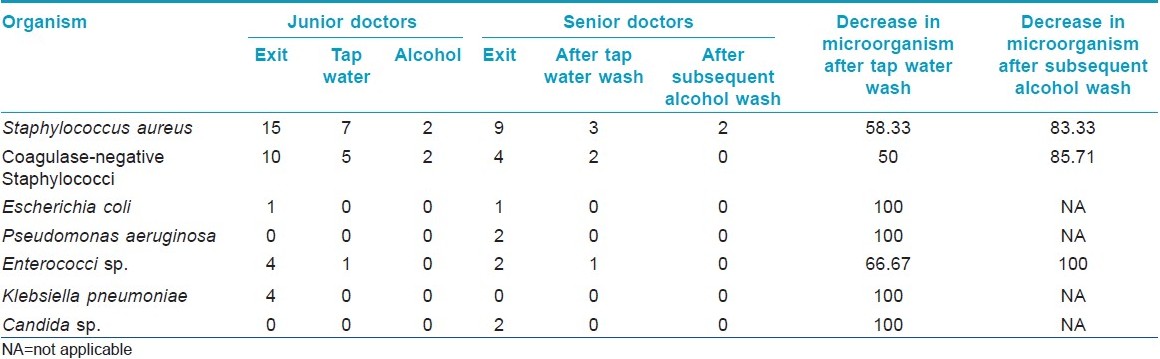
Among the 44 study subjects, 26 (59.1%) of the hand swabs were positive for a bacterium at entry, whereas 40 (90.9%) of the swabs were positive at exit from the wards [Figure - 1],[Table - 1]. All the 26 samples at entry grew one organism but, at exit, 14 (31.82%) showed the growth of two organisms. Staphylococcus topped the list both at entry (n = 26, 59.1%) and exit (n = 38, 86.36%). Coagulase-positive strains were overall the highest (n = 36, 40.91%) in number, although, at entry, coagulase-negative strains (n = 14, 31.81%) were mildly higher. At exit, the Staphylococcus aureus isolation rates were higher by 27.3% compared with that at entry. Gram negative strains (n = 14, 31.81%) appeared only at exit [Table - 1]. Fisher′s exact test showed a significant (P-value = 0.009) difference between Gram positive and negative organisms at exit compared with that at entry. Junior (n = 30, 68.2%) and senior doctors (n = 14, 31.8%) showed equal rates of contamination at entry (junior n = 20; senior, n = 6) and at exit (junior n = 25; senior n = 13). At entry, there was no significant difference in carriage rates of senior and junior doctors (P-value = 0.7921, Chi-square test). Also, at exit, the Fisher′s exact test showed no significant difference in the rates of contamination in the two groups (P-value = 0.0878). Analysis of data from different wards [Table - 2] showed that doctors exiting from both the Medicine and the Dermatology wards were liable to harbor a Gram negative organism (33% in emergency ward vs. 20% in chronic ward - Medicine; 35.3% in Dermatology). A Gram positive organism at exit was found in 82% (n = 14) of the cases in the Dermatology ward and in 88.8% (n = 24) of the cases in the Medicine wards (P = 0.8702; test for significance of proportions). Thus, there is not much difference in contamination of the two wards. At entry too, the rates of contamination are similar (59.2% in Medicine vs. 58% in Dermatology). However, in the medicine emergency wards, only one of 21 samples at exit were negative for an organism (4.76%), while in the chronic ward, negativity was seen in two of six samples (33.3%) (P = 0.11; Fisher′s exact test). Thus, doctors working in the emergency ward are liable to greater degrees of contamination. The antibiotic-sensitivity patterns were also similar in the two wards. After hand washing, repeat swabs showed a 76% decrease in the carriage rates with simple tap water washing and a further 16.5% decrease with alcohol hand washing [Table - 3]. Except for a few cases of Staphylococcus, all other strains were eliminated. After different hand wash techniques, Chi-square for trend showed no significant difference in the rate of decrease in coagulase-positive vs. coagulase-negative Staphylococcus (P-value = 0.9793) or Gram positive vs. Gram negative organisms. Also, there was no difference between senior and junior doctors.
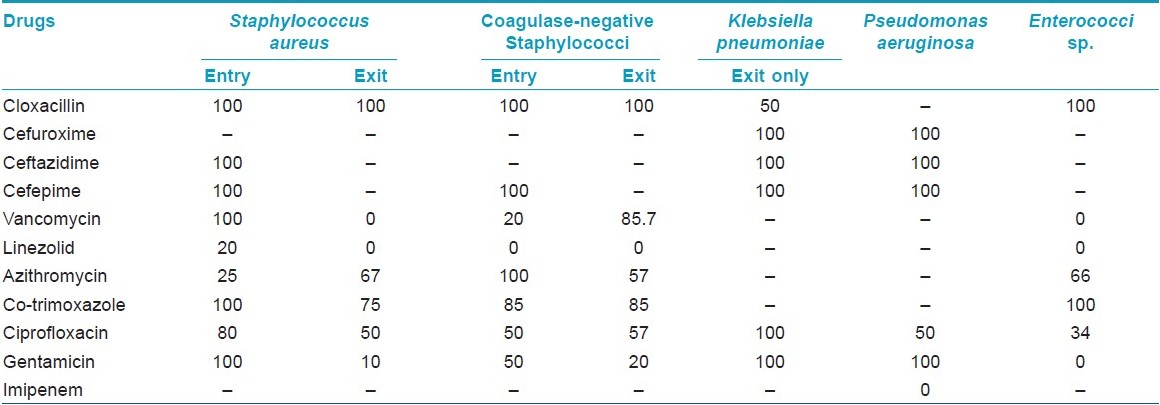
The antibiotic-sensitivity pattern shows that the organisms in the doctors′ hands were potentially multidrug resistant [Table - 4]. Analysis of the data showed that the beta-lactam group of drugs was mostly ineffective in this setting. Among the drugs targeted for Gram positive organisms, linezolid was mostly sensitive, but vancomycin showed significant resistance. Another significant observation was that the organisms at entry were more resistant to these drugs than those at exit. Among the low-cost drugs, only azithromycin retained some efficacy. Imipenem, the drug for virulent Gram negative organisms, was mostly effective.
Discussion
Hospital environment is a contaminated environment. Despite improvements in hygienic practices, there still remains much to be desired. Doctors inadvertently act as sources of infection to the patients. Common objects like dresses of hospital staff are often sources of infection. Often, both Gram positive and Gram negative bacteria are isolated.
A study from Brazil showed that even food objects are not spared. [5] The study revealed that the health care workers were directly linked to milk contamination, and public sectors were found to be more infected than the private ones. In our study, we found significant contamination rates with Staphylococcus.
In a hospital, the patients are living in a high-risk environment, where the objects around them are potential sources of infection. Nzeako et al.[6] performed a bacteriological study in Oman, where they tried to find the organisms in various objects in and around the patients. Their rate of positivity was 61% and the resistance pattern was also alarming.
A study from Delhi showed that food handlers in hospitals carried a large number of organisms in their hands, especially beneath their nails. [7] A large part was enteric organisms, proving poor personal hygiene.
Objects used by doctors, like mobile phones, are also contaminated from their hands and act as reservoirs of these bacteria later. [8] In a study from Turkey, the rate of contamination was more than 90% and, in more than 10% of the cases, the contamination was polymicrobial. [6] In the same study, the rate of MRSA and ceftazidime-resistant bacteria isolation from the hands of health care workers was also high. The same conclusion was drawn from a study in Sri Lanka, [9] when anesthetists were found to harbor bacteria like Staphylococcus. In our study, a large proportion of doctors (31.82% at exit) were found to harbor two organisms.
Healthcare workers′ compliance with hand washing is known to be poor, with doctors performing particularly badly. When the Department of Health published its handwashing guidance, a storm of correspondence in the BMJ excused low compliance on grounds of lack of time, poor availability of sinks and soaps, skin sensitivity and lack of evidence. [10] The present study would provide the evidence needed to incorporate the practice of hand washing in the health care workers. It is shown here that simple tap water hand washing reduces the contamination rate significantly (>50% for Staphylococcus and 100% for some Gram negative organisms).
Health care workers are notorious for their laxity in hand washing. [11] But, what such callousness can cause was shown by Peacock et al.[12] in 1980 by the rapid spread of MRSA in a hospital. Such nosocomial spreads can cause large burdens in terms of cost. In our study also, we found the isolated Staphylococci, both coagulase positive and coagulase negative, to be 100% resistant to the penicillin and cephalosporin group of drugs. Even drugs of other classes like fluoroquinolones and macrolides showed marked resistance (Staphylococcus: ciprofloxacin 80% resistance at entry).
Even infants are not safe. Brown et al.[13] performed a study that showed that pediatric wards are also contaminated by doctors as are adult patient wards. In the wake of the Staphylococcal epidemics of the 1950s, Rammelkamp and co-workers [14] demonstrated that direct contact, and not airborne transmission, was the main mode of transmission of Staphylococcus aureus. The need to reduce infection and hospital-acquired antimicrobial resistance prompted a systematic review of handwashing by Thames Valley University as part of the EPIC study. [15] This concluded that there was good evidence that direct patient contact resulted in hand contamination by pathogens. For example, 80% of the staff dressing wounds infected with MRSA carried the organism on their hands for up to 3 h. Immediate washing with liquid soap and water virtually eradicates the organism. [12],[16] An intensive therapy unit study showed that 40% of all patient-nurse interactions resulted in transmission of Klebsiella to the hands of healthcare workers, lasting up to 150 min, even after contact as slight as touching a patient′s shoulder. [17] A study of healthcare workers′ hands sampled within half an hour of contact with patients with Clostridium difficile infection showed contamination on nearly 60% of the hands, even after activities as simple as returning drug charts to the end of beds. Washing with soap and water virtually eradicated the organism. [18] In our study, a simple hand washing (first with water and then with alcohol) has shown to reduce the carriage rates significantly. The study has shown that doctors are carriers of a large number of pathogens. Many of the bacteria are multidrug resistant. These are potential sources of infection to the patients, especially the immunocompromised ones. Simple hand washing can decrease this contamination by a significant extent. Thus, better awareness is needed among the doctors. These types of studies are bound to make doctors self-conscious and they can exert an extra effort to clean their hands. But, still, a high rate of contamination is observed.
Acknowledgment
The authors would like to thank Dipanjan Bandyopadhyay, MD (Med), Associate Professor, Department of Medicine, who guided them throughout the study and also helped with the technical problems.
| 1. |
Ducel J, Fabry L, Nicolle G, editors. Prevention of hospital acquired infections: A practical guide. 2 nd ed. WHO report. Available from: http://www.who.int/csr/resources/publications/drugresist/WHO_CDS_CSR_EPH_2002_12/en/ . [Last accessed on 2010 August 10].
[Google Scholar]
|
| 2. |
Schaberg DR, Culver DH, Gaynes RP. Major trends in the microbial etiology of nosocomial infection. Am J Med 1991;91:S72-5.
[Google Scholar]
|
| 3. |
McAdam AJ, Onderdonk AB. Laboratory Diagnosis of Infectious Diseases. In: Fauci AS, Kasper DL, Longo DL, Braunwald E, Hauser SL, Jameson JL, et al. editors. Harrison′s Principles of Internal Medicine. 17 th ed. USA: McGraw Hill; 2008. p. e97-105.
[Google Scholar]
|
| 4. |
Bauer AW, Kirby WM, Sherris JC, Turck M. Antibiotic susceptibility testing by a standardized single disc method. Am J Clin Pathol 1966;45:493-6.
[Google Scholar]
|
| 5. |
Cairo RC, Silva LR, Andrade CF, Barberino MG, Bandeira AC, Santos KP, et al. Bacterial contamination in milk kitchens in pediatric hospitals in Salvador. Braz J Infect Dis 2008;12:217-21.
[Google Scholar]
|
| 6. |
Nzeako BC, Daughari HA, Lamki ZA, Rawas OA. Nature of bacteria found on some wards in Sultan Qaboos University Hospital, Oman. Br J Biomed Sci 2006;63:55-8.
[Google Scholar]
|
| 7. |
Malhotra R, Lal P, Prakash SK, Daga NK, Kishore J. Study of hand hygiene and enteroparasite infestation among food handlers working in a medical college of North India. Indian J Pathol Microbiol 2006:49:296-301.
[Google Scholar]
|
| 8. |
Ulger F, Esen S, Dilek A, Yanik K, Gunaydin M, Leblebicioglu H. Are we aware how contaminated our mobile phones with nosocomial pathogens? Ann Clin Microbiol Antimicrob 2009;8:7.
[Google Scholar]
|
| 9. |
Gunasekara TD, Kudavidanage BP, Peelawattage MK, Meedin F, Guruge LD, Nanayakkara G, et al. Bacterial Contamination of anaesthetists hands, personal mobile phones and wrist watches used during theatre. Sri Lanka J Anesthesiol 2009;17;11-5.
[Google Scholar]
|
| 10. |
Stone SP. Hand hygiene-the case for evidence-based education. J R Soc Med 2001;94:278-81.
[Google Scholar]
|
| 11. |
Sen R, Keaney M, Trail A, Howard C, Chadwick P. Hand washing healthcare workers washed their hands on only a third occasions. BMJ 1999;319:518.
[Google Scholar]
|
| 12. |
Peacock JE Jr, Marwick FJ, Wenzel RP. Methicillin-resistant Staphylococcus aureus: Introduction and spread within a hospital. Ann Intern Med 1980;93:526-32.
[Google Scholar]
|
| 13. |
Jeffrey B, Fretz F, Ann RN, Dennis L, James TK. High rate of hand contamination and low rate of hand washing before infant contact in a neonatal intensive care unit. Pediatr Infect Dis J 1996;5:908-10.
[Google Scholar]
|
| 14. |
Mortimer EA, Wolinsky E, Gonzaga AJ, Rammelkamp CH. Role of airborne transmission in staphylococcal infections. BMJ 1966;1:319-22.
[Google Scholar]
|
| 15. |
Pratt RJ, Pellowe C, Liveday HP, Robinson N, Smith GW, Barrett S, et al. The epic project: Developing national evidence-based guidelines for preventing healthcare associated infections. J Hosp Infect 2001;47:S3-82.
[Google Scholar]
|
| 16. |
Thompson RL, Cabezudo I, Wenzel RP. Epidemiology of nosocomial infections caused by methicillin resistant Staphylococcus aureus. Ann Intern Med 1982;1987:309-17.
[Google Scholar]
|
| 17. |
Casewell M, Phillips I. Hands as a route of transmission for Klebsiella species. BMJ 1977;2:1315-7.
[Google Scholar]
|
| 18. |
Samore MH, Venakartaraman L, De Girolami PC, Arbeit RD, Karchmer AW. Clinical and molecular epidemiology of sporadic and clustered cases of nosocomial Clostridium difficile diarrhea. Am J Med 1996;100:32-40.
[Google Scholar]
|
Fulltext Views
4,341
PDF downloads
2,633





