Translate this page into:
Beware of miconazole – warfarin interactions!
Correspondence Address:
Ajit Barve
7, Aradhana Adarsh Colony, Thane East, Maharashtra
India
| How to cite this article: Barve A. Beware of miconazole – warfarin interactions!. Indian J Dermatol Venereol Leprol 2020;86:702-703 |
Sir,
Nowadays, we commonly come across patients with tinea corporis involving extensive areas of the body surface. The commonly used topical antifungal miconazole can interact with warfarin. Efficacy as well as safety of warfarin, depend on the international normalized ratio. Guided by international normalized ratio levels, physicians need to modify warfarin dosage, factoring in variables like co-prescribed medications, dietary changes, concomitant acute illnesses, liver disease and alcohol intake.[1],[2] Miconazole inhibits CYP2C9 which is one of the major cytochrome P450 isozymes of warfarin metabolism. This causes decreased warfarin clearance and enhancement of its anticoagulant effect.[3]
The medicines and healthcare products regulatory agency (MHRA) of the United Kingdom has issued the following alert in this regard, advising patients on warfarin, against concurrent use of miconazole oral gel. 3 Up to April 13, 2016, medicines and healthcare products regulatory agency received 146 yellow cards that reported possible interactions between miconazole and warfarin. One hundred and twenty eight (88%) of these reports were regarding the oral gel form of miconazole; the reports comprised of raised international normalized ratio in 111 (76%) cases, contusion in 21 (14%) cases, hematuria in 17 (11%) cases, and epistaxis in 8 (5%) cases. International normalized ratio was raised above 10 in about half of these 146 reports and thus patients were at serious risk of a hemorrhagic event (target international normalized ratio range for a long-term warfarinized patient is generally between 2 and 3). The hemorrhagic episode resulting from miconazole-warfarin interaction was fatal in three cases.[3] Since that article in June 2016, the medicines and healthcare products regulatory agency received 25 more yellow card reports of miconazole-warfarin interaction.[4]
Numerous reports of miconazole oral gel and warfarin interactions are succinctly described by Pemberton.[5] Miconazole-warfarin interactions have also been reported with topical use of miconazole on the cutaneous surface and the vaginal mucosa [Table - 1]. Devaraj et al. have described an alarming rise in international normalized ratio from a range of '2.2 and 3.1' to 21.4 in an 80-year-old man on long-term warfarin, following topical miconazole application for 2 weeks on the groins.[6] Broos et al. have described four patients on coumarin drugs, who had applied miconazole in the perianal region, for intertrigo on inframammary area, for extensive tinea corporis on the groins and the armpits, with the resultant interaction.[7]
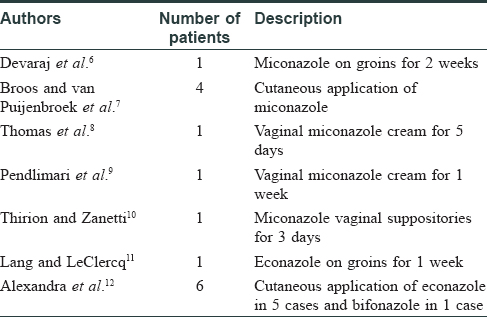
Miconazole-warfarin interactions following vaginal application of miconazole cream have been described.[8],[9] Application of miconazole cream for vaginal candidiasis for 1 week led to an international normalized ratio >13 and an intestinal intramural hematoma in one of the reports.[9] Thirion et al. have noted miconazole-warfarin interaction with miconazole vaginal suppositories. After a 3-day course of 200 mg miconazole vaginal suppositories for yeast infection, the patient who was on warfarin, had ecchymosis on the limbs and a rise in international normalized ratio to 9.77 from 2.69.[10]
Apart from miconazole, the potential for interaction exists between topical econazole and warfarin. Concomitant administration of topical econazole and warfarin has resulted in an enhancement of the anticoagulation effect, especially when econazole was used on an occluded area, or on a large body surface area. Monitoring of international normalized ratio and prothrombin time is advisable in such cases.[13]
Lang et al. described a 79-year-old physician, on long-term warfarin therapy, with international normalized ratio stable in the range of 1.8 to 3. Within the first week of application of econazole nitrate for candidiasis on the groins, he had ecchymosis and international normalized ratio had risen to 12. However, the same patient had previously used topical miconazole without a similar effect.[11] Enhancement of anticoagulation in six patients receiving coumarin and topical azoles (econazole [five cases] and bifonazole [one case]) was described by Alexandra et al.[12]
Currently, luliconazole is a very commonly prescribed topical antifungal in India. A PubMed search with the terms “luliconazole warfarin interaction” did not return any case reports on the topic. Further, the luliconazole product label accessed via the United States Food and Drug Administration website did not reveal any mention of such an interaction.[14] However, in the current scenario, where luliconazole may be applied on an extensive body surface area, due caution must be exercised.
On intact skin, percutaneous absorption of topical azoles is generally less than 1%. The absorption may rise to 4% on inflamed or damaged skin.[15] When topical drugs are applied to a large body surface area, the systemic absorption increases. In India, we are facing a situation of 'antifungal treatment failure', for cutaneous dermatophytoses, and commonly see patients with extensive body surface area involvement, suffering from tinea corporis.
This communication highlights the potential dangers of using topical miconazole or econazole in patients on warfarin, and dermatologists must be aware of it.
To best manage patients who are on warfarin, the following pointers may be considered:
- Complete drug history must be taken.
- Dermatologists must be abreast of the latest literature regarding drug interactions.
- It is best to manage such patients in coordination with the physician prescribing warfarin, so that appropriate monitoring of the patient is possible.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Hirsh J, Dalen JE, Anderson DR, Poller L, Bussey H, Ansell J, et al. Oral anticoagulants: Mechanism of action, clinical effectiveness, and optimal therapeutic range. Chest 1998;114:445S-69.
[Google Scholar]
|
| 2. |
Holbrook AM, Pereira JA, Labiris R, McDonald H, Douketis JD, Crowther M, et al. Systematic overview of warfarin and its drug and food interactions. Arch Intern Med 2005;165:1095-106.
[Google Scholar]
|
| 3. |
Available from: https://www.gov.uk/drug-safety-update/topical-miconazole-including-oral-gel-reminder-of-potential- for-serious-interactions-with-warfarin#latest-mhra-review. [Last accessed on 2018 Jun 17].
[Google Scholar]
|
| 4. |
Available from: https://www.gov.uk/drug-safety-update/miconazole-daktarin-over-the-counter-oral-gel-contraindicated- in-patients-taking-warfarin. [Last accessed on 2018 Jun 17].
[Google Scholar]
|
| 5. |
Pemberton MN. Nystatin and miconazole: Pharmacological and clinical evidence regarding interactions with warfarin. Oral Dis 2016;22:761-5.
[Google Scholar]
|
| 6. |
Devaraj A, O'Beirne JP, Veasey R, Dunk AA. Interaction between warfarin and topical miconazole cream. BMJ 2002;325:77.
[Google Scholar]
|
| 7. |
Broos N, van Puijenbroek EP. Interaction between topical miconazole and coumarins. Eur J Clin Pharmacol 2010;66:1171-2.
[Google Scholar]
|
| 8. |
Thomas JL, Dunn D, Pelletier A, Franks AS. Hyperprothrombinaemia as a result of a possible warfarin and intravaginal miconazole interaction. South Med J 2010;103:1063-5.
[Google Scholar]
|
| 9. |
Pendlimari R, Anaparthy R, Sugumar A. Drug interaction presenting as acute abdomen. World J Gastrointest Pharmacol Ther 2010;1:40-2.
[Google Scholar]
|
| 10. |
Thirion DJ, Zanetti LA. Potentiation of warfarin's hypoprothrombinemic effect with miconazole vaginal suppositories. Pharmacotherapy 2000;20:98-9.
[Google Scholar]
|
| 11. |
Lang PG Jr, LeClercq AH. Increase in anticoagulant effect of warfarin in a patient using econazole cream. J Am Acad Dermatol 2006;55:S117-9.
[Google Scholar]
|
| 12. |
Alexandra JF, Pautas E, Gouin-Thibault I, Siguret V, Loriot MA. Overanticoagulation with coumarin and cutaneous azole therapy. Ann Intern Med 2008;148:633-5.
[Google Scholar]
|
| 13. |
Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/018751s025lbl.pdf. [Last accessed on 2018 Jul 22].
[Google Scholar]
|
| 14. |
Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/204153s004s005lbl.pdf20. [Last accessed on 2020 Mar 30].
[Google Scholar]
|
| 15. |
Ritter W. Pharmacokinetics of azole compounds. In: Berg D, Plempel D, editors. Sterol Biosynthesis Inhibitors. Chichester: Ellis Horwood; 1988. p. 398-429.
[Google Scholar]
|
Fulltext Views
5,120
PDF downloads
2,977





