Translate this page into:
Black grain eumycetoma of the breast misdiagnosed as fibroadenoma
2 Department of Obstetrics and Gynecology, SMS Medical College and Attached Hospitals, Jaipur, Rajasthan, India
3 Department of Pathology, SMS Medical College and Attached Hospitals, Jaipur, Rajasthan, India
Correspondence Address:
Sunil K Kothiwala
Department of Dermatology and Venereology, SMS Medical College and Attached Hospitals, Jaipur, Rajasthan
India
| How to cite this article: Kothiwala SK, Purohit S, Meena M, Jindal A. Black grain eumycetoma of the breast misdiagnosed as fibroadenoma. Indian J Dermatol Venereol Leprol 2015;81:521-523 |
Sir,
Mycetoma is a chronic subcutaneous localized infection characterized by the triad of swelling, discharging sinuses and discharge of grains that may be caused by bacteria (actinomycetoma) or fungi (eumycetoma). Although it most commonly involves the foot, lower leg or hand, involvement of the head or back may also occur. [1] The incidence is low in other parts of the body because of the lower risk of trauma as the initiating factor. We report an adult woman with multiple nodules in the breast misdiagnosed as fibroadenoma and later proved to be eumycetoma.
A 26-year-old woman presented with multiple asymptomatic subcutaneous nodules in the right breast for 4 years. Initially she developed a solitary asymptomatic subcutaneous nodule in the right inferior-medial quadrant near the areola, followed by a similar lesion in the central quadrant within the next 6 months that was diagnosed as fibroadenoma by a surgeon. Ultrasonography revealed two sonolucent areas of 25.2 × 11.5 mm and 20.7 × 9.3 mm in the infero-medial and central quadrants, respectively in the peri-areolar region. Excision biopsy of the small central nodule showed fibrocystic disease. The larger inferior-medial nodule was then excised. The patient continued to develop new lesions until she presented to us with multiple, non-tender, coalescing soft to firm, subcutaneous nodules in the infero-lateral side of the breast that varied from 2 to 4 cm in size. The overlying skin was intact except for the scars of previous excisions [Figure - 1]a. No discharging grains or sinuses were seen. Excision biopsy of the nodules revealed multiple black grains between nodules surrounded by fat globules [Figure - 1]b. The grains were sent for fungal culture, while the intact nodule was sent for histopathology.
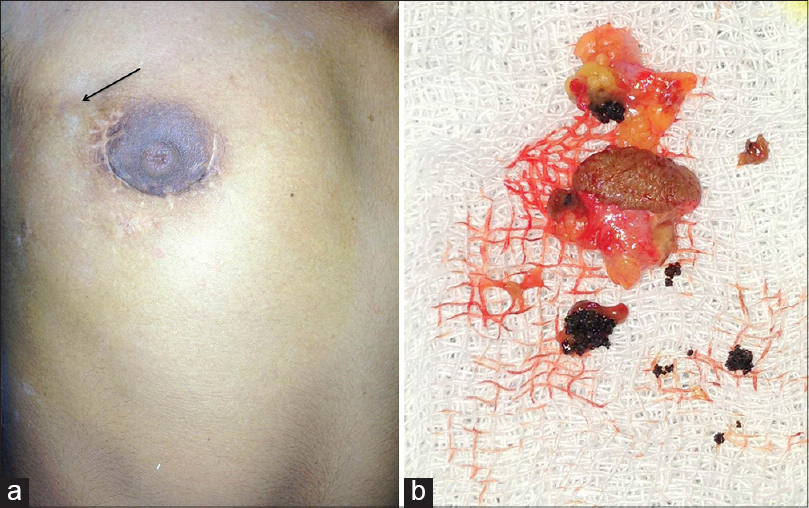 |
| Figure 1: (a) Scar mark of previous excision biopsy and surgery over right breast around areola. Arrow indicates site of current excision biopsy from subcutaneous nodule and (b) Multiple black-brown hard grains surrounded by fat tissue on excision biopsy from nodule |
Histopathology from the excised nodule showed a chronic granulomatous inflammatory reaction with neutrophil abscesses and scattered giant cells [Figure - 2]a and b. The center of the nodule showed round to oval shaped grains, variable in size and containing fungal colonies comprised of broad, non-septate hyphae with round, vesiculated chlamydoconidia embedded in a brown cemented matrix [Figure - 3]a. Many fungal hyphae were also seen on Gomori methenamine silver stain [Figure - 3]b. Fungal culture did not grow any organism even after 4 weeks. Based on the color of grains, histopathology and special stains, we diagnosed eumycetoma of the breast. We treated her with itraconazole, 200 mg twice a day. There was no significant change after 15 days of treatment and she was subsequently lost to follow-up.
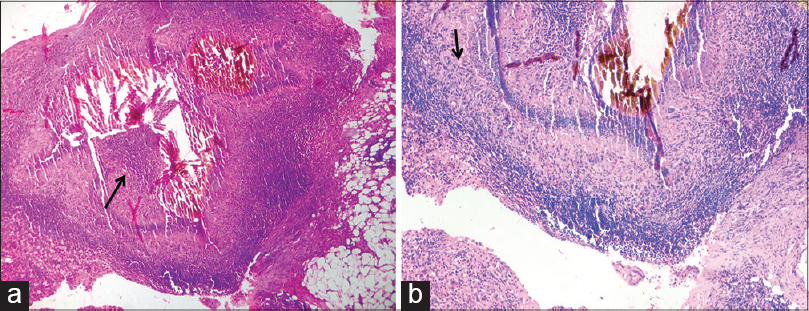 |
| Figure 2: (a) Area of suppuration with grains in center ×100 and (b) surrounded by chronic granulomatous inflammatory infiltrate ×200. Hematoxylin and eosin |
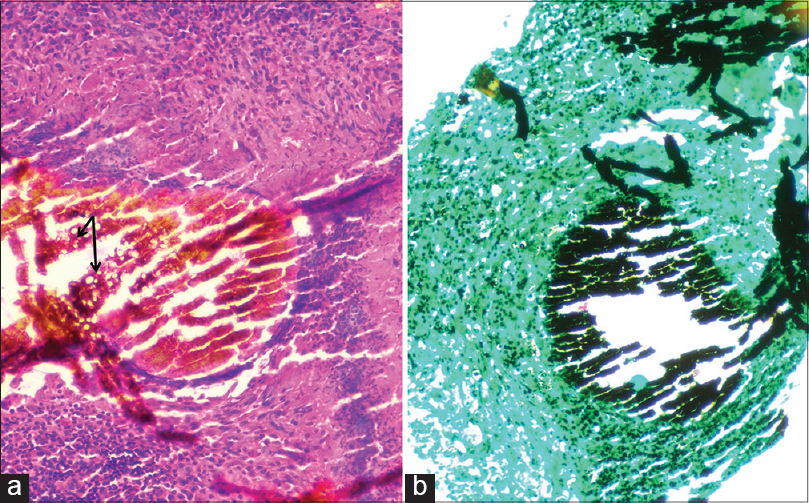 |
| Figure 3: Round to oval shaped grains, variable in size and containing fungal colonies comprised of broad, nonseptate hyphae with round, vesiculated chlamydoconidia embedded in a brown, cemented matrix. (a) Hematoxylin and eosin, ×400 and (b) Grocott's methenamine silver stain, ×200 |
Mycetoma usually involves parts of the body that come in contact with soil during daily activities. [2] The foot is the most commonly affected site, being involved in 70% of patients while the hand, the next most commonly affected area, is involved in 12% of patients. Less frequently, the knee, arm, leg, head and neck, [3] inguinal area [4] and the perineum, [5] are affected in descending order of frequency. Rarely, sites such as the chest [6] and abdominal walls, [7] facial bones, mandible, [8] testes, [9] vulva, [10] paranasal sinuses [11] and eyelids [12] may be affected. For poorly understood reasons, actinomycetomas are more frequent than eumycetomas at uncommon locations such as the abdominal wall, chest, head and neck. Maiti, et al. showed that mycetomas that occurred on covered parts of the body significantly differed from mycetomas occurring in exposed areas and the former were almost always actinomycetomas. [2]
The diagnosis of eumycetoma in our case was based on the black color of grains with fungal colonies, which were embedded in a brown matrix as seen on histopathology and Gomori methenamine silver stain. On histopathology, it is difficult to differentiate between eumycetoma and actinomycetoma unless a grain is seen, as the host reaction is similar. An inflammatory nodule typically centers on an area of suppuration containing numerous polymorphonuclear cells with or without granulomatous inflammatory reaction in the dermis and subcutaneous tissue. When grains are noted, eumycotic colonies are more frequently surrounded by fibrotic tissue than in actinomycetoma. Furthermore, the shape, size, macroscopic color and affinity for stains of the grains are useful in preliminary characterization of the mycetoma. In contrast to actinomycetoma granules that are 20 μm to 4 mm in size, eumycotic mycetomas usually have larger grains, up to 5 mm in diameter, composed of fungal hyphae that are septate, 2-6 μm thick, often with distorted shapes, and spherical, thick-walled, terminal chlamydoconidia. The presence of an amorphous matrix narrows the diagnosis to consideration of three eumycotic agents, Madurella mycetomatis, Madurella grisea and Leptosphaeria. If this amorphous matrix is present throughout the colony imparting a grainy appearance it may suggest M. mycetomatis.
This presentation of eumycetoma as coalescing, subcutaneous, breast nodules unaccompanied by any surface changes such as discharging sinuses and grains in spite of disease lasting 4 years is distinctly unusual.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Fahal AH. Mycetoma thorn on the flesh Review article. Trans R Soc Trop Med Hyg 2004;98:3-11.
[Google Scholar]
|
| 2. |
Maiti PK, Haldar PK. Mycetomas in exposed and non-exposed parts of the body: A study of 212 cases. Indian J Med Microbiol 1998;16:19-22.
[Google Scholar]
|
| 3. |
Gumaa SA, Mahgoub ES, E Sid MA. Mycetoma of the head and neck. Am J Trop Med Hyg 1986;35:594-600.
[Google Scholar]
|
| 4. |
Dogra D, Ramam M, Banerjee U. Inguinal mycetoma. Acta Dermatol Venereol 1996;76:414.
[Google Scholar]
|
| 5. |
Gupta S, Jain K, Parmar C, Shah P, Raval RC. Mycetoma: Nonvenereal perineal lesions. Indian J Sex Transm Dis 2010;31:39-41.
[Google Scholar]
|
| 6. |
Shafei H, McCormick CS, Donnelly RJ. Madura foot of the chest wall; cure after radical excision. Thorac Cardiovasc Surg 1992;40:198-200.
[Google Scholar]
|
| 7. |
Fahal AH, Suliman SH, Gadir AF, EL Hag IA, EL Amin FI, Gumaa SA, et al. Abdominal wall mycetoma: An unusual presentation. Trans R Soc Trop Med Hyg 1994;88:78-80.
[Google Scholar]
|
| 8. |
Gumaa SA, Satir AA, Shehata AH, Mahgoub ES. Tumor of the mandible caused by Madurella mycetomil. Am J Trop Med Hyg 1975;24:471-4.
[Google Scholar]
|
| 9. |
Clarke PR. Mycetoma of the testis. Lancet 1953;2:1341.
[Google Scholar]
|
| 10. |
Fahal AH, Sharfy AR. Vulval mycetoma: A rare cause of bladder outlet obstruction. Trans R Soc Trop Med Hyg 1998;92:652-3.
[Google Scholar]
|
| 11. |
Klossek JM, Serrano E, Péloquin L, Percodani J, Fontanel JP, Pessey JJ. Functional endoscopic sinus surgery and 109 mycetomas of the paranasal sinuses. Laryngoscope 1997;107:112-7.
[Google Scholar]
|
| 12. |
Alridge J, Kirk R. Mycetoma of the eyelid. Br J Ophthalmol 1940;24:211-2.
[Google Scholar]
|
Fulltext Views
3,289
PDF downloads
2,794





