Translate this page into:
Bullous pemphigoid and antecedent neurological diseases: An association with dementia
2 National Skin Centre, Singapore
3 National Skin Centre; Department of Dermatology, Lee Kong Chian School of Medicine; Department of Dermatology, Tan Tock Seng Hospital, Singapore
Correspondence Address:
Hong Liang Tey
National Skin Centre, 1 Mandalay Road, 308205
Singapore
| How to cite this article: Yu Phuan CZ, Yew YW, Tey HL. Bullous pemphigoid and antecedent neurological diseases: An association with dementia. Indian J Dermatol Venereol Leprol 2017;83:457-461 |
Abstract
Background: Bullous pemphigoid is the most common subepidermal immunobullous disorder. Studies have reported the association between bullous pemphigoid and various neurological diseases.Aims: The aim of this study was to evaluate whether bullous pemphigoid is associated with pre-existent neurological diseases and whether specific diseases exhibit this association.
Methods: All dermatology inpatients from January 2010 to May 2015 were analyzed. Bullous pemphigoid cases were identified based on clinical features and consistent histopathologic and direct immunofluorescence findings. Patients with other autoimmune bullous skin disorders were excluded. An equal number of inpatients with other skin conditions were selected randomly as age- and sex- matched controls.
Results: Out of 3015 inpatients, 103 cases of bullous pemphigoid and 103 age- and sex-matched controls were included. Seventy six patients with bullous pemphigoid had a history of at least one neurological disease. After adjusting for age, gender, race, functional status and neuro-psychiatric medications, patients with bullous pemphigoid were found to be approximately thrice as likely to have a history of at least one neurological disease than were controls (odds ratio: 2.88; 95% confidence interval: 1.32–6.26; P = 0.008). Amongst the pre-existing neurological diseases, only dementia was statistically more prevalent in bullous pemphigoid cases compared to controls (adjusted odds ratio: 2.61; 95% confidence interval: 1.19–5.75; P = 0.017). Parkinson disease and psychiatric disorders demonstrated a higher adjusted risk among bullous pemphigoid patients but the difference was not statistically significant.
Limitations: The limitations were potential referral and selection bias, as the patients were inpatients. There is a possible misclassification as the diagnosis of neurological diseases was performed using medical records. The duration from the diagnosis of neurological diseases to bullous pemphigoid could not be accurately determined as it was a retrospective review of records and most neurological diseases have a prolonged course.
Conclusions: Pre-existent neurological disease, specifically dementia, was found to be associated with bullous pemphigoid.
Introduction
Bullous pemphigoid typically affects the elderly, accounting for 88% of all the cases of subepidermal immunobullous disorders at our center.[1],[2] In Singapore, the annual incidence rate of bullous pemphigoid is approximately, 7.6 cases per million population with a mean age at diagnosis of 77 years.[2]
Several studies have described an association of bullous pemphigoid with neurological conditions, such as hemiplegia and chronic intake of neuroleptics and aldosterone antagonists, suggesting that immobility, muscle weakness or dependency may influence the development of bullous pemphigoid.[3],[4],[5],[6],[7] Currently, Asian epidemiological studies are lacking; most available ones are small, devoid of controls and do not assess neurological diseases as preceding or following diagnosis of bullous pemphigoid.[8],[9] Our study aims to determine whether pre-existing neurological diseases are associated with bullous pemphigoid development.
Methods
In this retrospective case–control study, the study population consisted of all inpatients with a confirmed diagnosis of bullous pemphigoid who were referred to the dermatology service at the Tan Tock Seng Hospital, Singapore between January 2010 and May 2015. The diagnosis of bullous pemphigoid was based on the presence of all of the following criteria: (1) Typical clinical features of subepidermal blisters affecting various parts of the body, as assessed by dermatologists, and consistent findings on (2) histology and (3) direct immunofluorescence. Age- and sex-matched controls in a 1:1 ratio were randomly selected from inpatients with other dermatological conditions admitted during the same period. Patients who had other autoimmune bullous skin conditions (such as pemphigus vulgaris and pemphigus foliaceus) and autoimmune connective tissue diseases (such as lupus and dermatomyositis) were excluded from the study.
Records of all patients and controls were evaluated for a past medical history of any neurological diseases when they were first diagnosed with bullous pemphigoid. Neurological diseases evaluated included dementia, stroke, Parkinson disease, epilepsy and psychiatric disorders. Confirmation of the neurological diagnoses was made using the International Classification of Disease diagnosis codes in the patients' medical records, as well as appropriate medications being prescribed for the respective neurological conditions. The diagnoses were made by geriatricians and neurologists based on clinical features, evidence of impairments in daily living and education-adjusted mini–mental state examinations, after exclusion of secondary causes.
Variables included in the analysis as possible confounding factors were age, gender, racial group, functional disability and medications prescribed for neuro-psychiatric diseases. Other comorbidities previously reported to be associated with bullous pemphigoid, such as ischemic heart disease, diabetes mellitus and internal malignancies, were also included in the analysis. Racial groups were categorized into “Chinese,” “Malay,” “Indians,” “Caucasians” and “other races.” Functional status was categorized into “independent,” “assisted,” “wheelchair-bound” or “bed-bound.” Comorbid conditions were determined based on the International Classification of Disease diagnosis codes in the patients' medical records.
The relationship between bullous pemphigoid and a medical history of neurological diseases was analyzed using the Chi-square or Fisher's exact tests. Multivariate logistic regression analysis was performed with bullous pemphigoid as the dependent variable, and age, gender, racial groups, functional status, neuro-psychiatric medications (such as anti-Parkinsonian, antiepileptic, antidepressant and antipsychotic drugs), ischemic heart disease, diabetes mellitus and internal malignancies as the independent variables. Multicollinearity between the independent variables was assessed using the variation inflation factor.
The Statistical Package for Social Sciences (IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY: IBM Corp.) was utilized for the analysis of the above-mentioned variables to evaluate statistical significance. Odds ratio, 95% confidence interval and P values were calculated to test the null hypotheses of the association between bullous pemphigoid with a history of neurological diseases. A two-sided P< 0.05 was considered statistically significant. The study was approved by our institution's Ethics Review Board.
Results
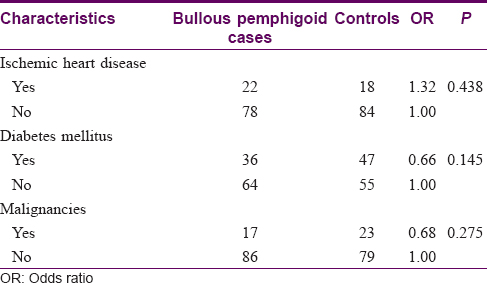
A total of 3015 inpatients were referred to the dermatology service during the study period. of which 103 cases of bullous pemphigoid and they 103 age and sex-matched controls were included in the study. The demographic characteristics and comorbidities of cases and controls are shown in [Table - 1] and [Table - 2]. Their mean age was 78.2 ± 11.4 years, and there was a slight female predominance of 51.0%. The majority of the patients were Chinese (84.0%), followed by Indians (4.4%). While most patients (35%) were functionally independent, a significant proportion (28.6%) of the patients was bed-bound. Bullous pemphigoid patients were about five times more likely to be bed-bound compared to controls (odds ratio: 5.26; 95% confidence interval: 2.49–11.15; P< 0.001). Eighty three (36%) patients had diabetes mellitus, 40 (22%) had a history of ischemic heart disease while 17 (16.5%) had a history of internal malignancies. Mean age, gender, race, risk of ischemic heart disease, diabetes mellitus and malignancies were similar between the two groups.
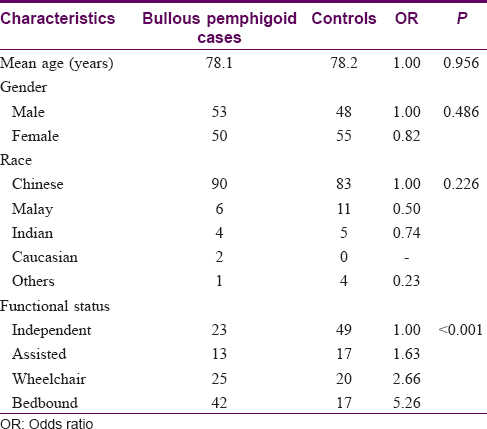
Of the 103 patients with bullous pemphigoid, there were 76 (73.8%) patients with a history of at least one neurological disease. Patients with bullous pemphigoid were found to be around three times more likely to have a history of at least one neurological disease compared to controls (odds ratio: 3.10; 95% confidence interval: 1.73–5.57; P< 0.001) [Table - 3]. Specifically, these patients had a higher prevalence of dementia, stroke, Parkinson disease, epilepsy and psychiatric disorders compared to controls.
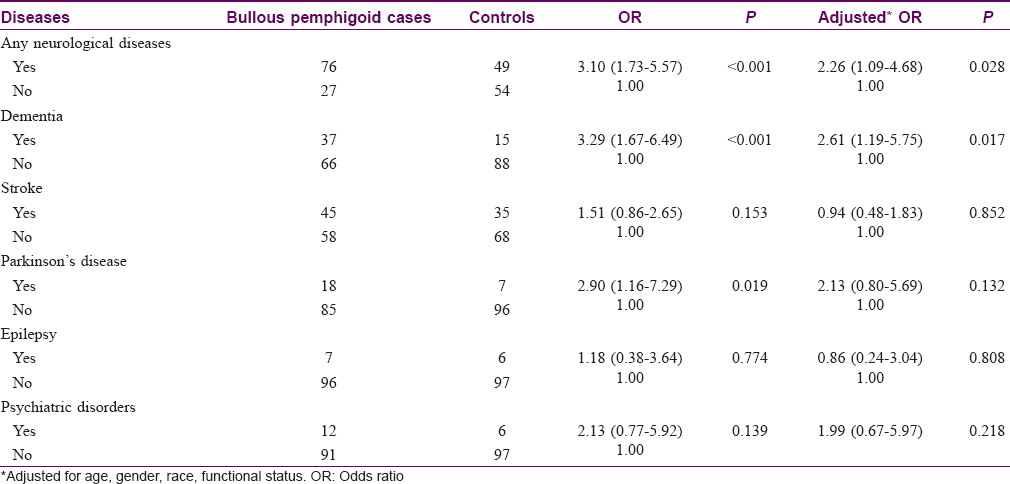
After adjusting for age, gender, race, functional status and any prescribed relevant neurological medications, there was a statistically significant increase in risk only for neurological diseases as a whole and dementia. Among those diagnosed with dementia, 10 (29.7%) had vascular dementia, 2 (5.4%) had mixed dementia and 25 (64.8%) had Alzheimer's dementia. The adjusted risks of Parkinson disease and psychiatric disorders remained higher among bullous pemphigoid patients, but these were not statistically significant. In the multivariate logistic regression model, the risks of being bed-bound among patients with bullous pemphigoid remained significantly higher compared to controls (odds ratio: 6.02; 95% confidence interval: 2.33–15.60; P< 0.001). Sensitivity analysis using different logistic regression analysis models yielded similar results. There was no evidence of mutli-collinearity between the independent variables in the models.
Discussion
This is a case–control study evaluating the association of pre-existing neurological diseases and bullous pemphigoid in an Asian population. Our study showed that there is a significantly higher prevalence of antecedent neurological diseases among bullous pemphigoid patients. Dementia was the only neurological disease found to have a significant association, after adjusting for confounding factors. In previous observational studies dementia, stroke, Parkinson disease, epilepsy and multiple sclerosis have been variably reported to be associated with bullous pemphigoid.[2],[3],[4],[7],[10],[11],[12] A similar study from England showed a significant association of bullous pemphigoid with cerebrovascular disease (adjusted odds ratio: 6.0; 95% confidence interval: 2.6–13.6) and dementia (adjusted odds ratio: 7.9; 95% confidence interval: 1.7–37.3).[11] In a recent study from Taiwan, an association was found between bullous pemphigoid and stroke (odds ratio: 2.37; 95% confidence interval: 1.78–3.15).[13] Another study conducted in Taiwan found that patients with bullous pemphigoid had a 3.62-fold increased risk for having neurological disease prior to the diagnosis of bullous pemphigoid.[7]
It is known that bullous pemphigoid and neurological diseases are more commonly seen in the older age groups. Degenerative neuronal diseases may induce autoimmune triggers against neuronal bullous pemphigoid antigen 1 (BP1) which may cross-react with cutaneous bullous pemphigoid antigen 1, manifesting as bullous pemphigoid in patients.[12] A recent study reports an association between degenerative neurological disease in mice and the dystonin gene which encodes for the neuronal isoform of bullous pemphigoid antigen 1.[14] Another study showed that knockout mice for the neuronal isoform of bullous pemphigoid antigen 1 developed severe neurodegeneration and dystonia due to the accumulation of intermediate filaments in motor neurons.[15] It has, therefore, been hypothesized that an autoimmune response initially directed against a neuronal isoform of bullous pemphigoid antigen 1 encoded by the dystrophin gene may secondarily trigger an autoimmune response against the epithelial isoform of bullous pemphigoid antigen 1.[13] In a recent study in bullous pemphigoid, it was found that high serum antibody levels against bullous pemphigoid antigen 1 correlated with the presence of neurological diseases.[16]
Previous studies have provided evidence that antecedent neurological diseases may lead to the development of bullous pemphigoid. Magnetic resonance imaging showed that in the acute phase of neurological pathologies, there is serious damage to the hematoencephalic barrier.[17],[18],[19] Pre-existing neurological diseases, such as Parkinson disease and multiple sclerosis, can trigger the exposure of neuronal isoforms which could potentially activate immune reactions and immunological cross-reactions, causing cutaneous damage.[20] It has been shown that there is an increase in the odds of developing bullous pemphigoid in people with neurological disease diagnosed more than 12 months prior with a 3-fold increase for dementia and Parkinson disease and a 2-fold increase for stroke and epilepsy.[12] Furthermore, a study done in England has shown that when accurate data were present on the time of onset of neurological disease, bullous pemphigoid followed neurological diseases in 85% of their patients with a median interval of 5.5 years.[11] However, most neurological diseases, especially dementia, occur slowly and progressively over time, and it is difficult to determine the exact date of disease incidence.
Some observational studies have evaluated physical trauma, decubital lesions or paralysis as risk factors for the onset of bullous pemphigoid.[3],[4],[5],[21],[22] The results were however, not conclusive. A hypothesis is that damage to the subepidermis from chronic pressure and subsequent antigen exposure can lead to an immune reaction triggering the onset of bullous pemphigoid.[20] Our study has similarly found an increasing level of functional dependency in bullous pemphigoid cases compared to controls [Table - 1]; however, this may be secondary to the increased prevalence of neurological diseases among the cases.
Bullous pemphigoid not uncommonly presents first with pruritus or non-specific erythematous patches and urticarial lesions in the early stages, while tense blisters develop weeks to months later.[23],[24],[25],[26] In a Swiss population study, 20% of 160 patients diagnosed with bullous pemphigoid did not have any primary skin lesions.[9] This implies that the diagnosis of bullous pemphigoid may often be missed in the early stages, during which treatment could have significantly reduced the morbidity of the disease. From our study's results, we opine that when patients present with early BP, during which the clinical signs are not uncommonly non-specific, the presence of neurological diseases as a risk factor serve to increase physicians' index of suspicion for the disease and closer monitoring for the evolution of the rashes into blisters can be instituted. Early intervention and treatment of bullous pemphigoid may significantly reduce the morbidity associated with the disease.
This is a matched case–control study with respect to age and gender with a logistical regression model being used to adjust for multiple confounding factors. However, there are some limitations in this study. There could be potential referral and selection bias since our study population consisted of inpatients in a general hospital. The control population may have a higher prevalence of neurological diseases or other comorbidities, and this could have a bias toward the null. While the diagnoses of bullous pemphigoid among our cases were made with typical clinical features assessed by dermatologists and further confirmed by histology and direct immunofluorescence findings, the diagnoses of neurological disease were made from medical records based on diagnosis codes and typical neurological medications. This could have led to misclassification. Our study did not have adequate numbers to evaluate the effect of some specific medications such as spironolactone as their chronic use has previously been reported to be associated with bullous pemphigoid.[22]
Conclusions
We found an association between the occurrence of bullous pemphigoid and antecedent neurological diseases, specifically dementia. When elderly patients present with nonspecific dermatological signs suspicious of early bullous pemphigoid, a history of neurological disease raises the index of suspicion, and serological tests can be performed to enable expedient diagnosis of the disease.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Bernard P, Vaillant L, Labeille B, Bedane C, Arbeille B, Denoeux JP, et al. Incidence and distribution of subepidermal autoimmune bullous skin diseases in three French regions. Bullous Diseases French Study Group. Arch Dermatol 1995;131:48-52.
[Google Scholar]
|
| 2. |
Wong SN, Chua SH. Spectrum of subepidermal immunobullous disorders seen at the National Skin Centre, Singapore: A 2-year review. Br J Dermatol 2002;147:476-80.
[Google Scholar]
|
| 3. |
Long CC, Lever LR, Marks R. Unilateral bullous pemphigoid in a hemiplegic patient. Br J Dermatol 1992;126:614-6.
[Google Scholar]
|
| 4. |
Foureur N, Descamps V, Lebrun-Vignes B, Picard-Dahan C, Grossin M, Belaich S,et al. Bullous pemphigoid in a leg affected with hemiparesia: A possible relation of neurological diseases with bullous pemphigoid? Eur J Dermatol 2001;11:230-3.
[Google Scholar]
|
| 5. |
Bunker CB, Brown E. Unilateral bullous pemphigoid in a hemiplegic patient. Br J Dermatol 1993;129:502.
[Google Scholar]
|
| 6. |
Bastuji-Garin S, Joly P, Picard-Dahan C, Bernard P, Vaillant L, Pauwels C, et al. Drugs associated with bullous pemphigoid. A case-control study. Arch Dermatol 1996;132:272-6.
[Google Scholar]
|
| 7. |
Chen YJ, Wu CY, Lin MW, Chen TJ, Liao KK, Chen YC, et al. Comorbidity profiles among patients with bullous pemphigoid: A nationwide population-based study. Br J Dermatol 2011;165:593-9.
[Google Scholar]
|
| 8. |
Di Zenzo G, Della Torre R, Zambruno G, Borradori L. Bullous pemphigoid: From the clinic to the bench. Clin Dermatol 2012;30:3-16.
[Google Scholar]
|
| 9. |
Kwan Z, Lai YN, Ch'ng CC, Tan AH, Tan LL, Robinson S, et al. The association between bullous pemphigoid and neurological disorders in a selected Malaysian population. Med J Malaysia 2015;70:81-5.
[Google Scholar]
|
| 10. |
Cordel N, Chosidow O, Hellot MF, Delaporte E, Lok C, Vaillant L, et al. Neurological disorders in patients with bullous pemphigoid. Dermatology 2007;215:187-91.
[Google Scholar]
|
| 11. |
Taghipour K, Chi CC, Vincent A, Groves RW, Venning V, Wojnarowska F. The association of bullous pemphigoid with cerebrovascular disease and dementia: A case-control study. Arch Dermatol 2010;146:1251-4.
[Google Scholar]
|
| 12. |
Langan SM, Groves RW, West J. The relationship between neurological disease and bullous pemphigoid: A population-based case-control study. J Invest Dermatol 2011;131:631-6.
[Google Scholar]
|
| 13. |
Yang YW, Chen YH, Xirasagar S, Lin HC. Increased risk of stroke in patients with bullous pemphigoid: A population-based follow-up study. Stroke 2011;42:319-23.
[Google Scholar]
|
| 14. |
Brown A, Bernier G, Mathieu M, Rossant J, Kothary R. The mouse dystonia musculorum gene is a neural isoform of bullous pemphigoid antigen 1. Nat Genet 1995;10:301-6.
[Google Scholar]
|
| 15. |
Guo L, Degenstein L, Dowling J, Yu QC, Wollmann R, Perman B, et al. Gene targeting of BPAG1: Abnormalities in mechanical strength and cell migration in stratified epithelia and neurologic degeneration. Cell 1995;81:233-43.
[Google Scholar]
|
| 16. |
Gambichler T, Segert H, Höxtermann S, Schmitz L, Altmeyer P, Teegen B. Neurological disorders in patients with bullous pemphigoid: Clinical and experimental investigations. J Eur Acad Dermatol Venereol 2015;29:1758-62.
[Google Scholar]
|
| 17. |
Kermode AG, Tofts PS, Thompson AJ, MacManus DG, Rudge P, Kendall BE, et al. Heterogeneity of blood-brain barrier changes in multiple sclerosis: An MRI study with gadolinium-DTPA enhancement. Neurology 1990;40:229-35.
[Google Scholar]
|
| 18. |
Barnes D, Munro PM, Youl BD, Prineas JW, McDonald WI. The longstanding MS lesion. A quantitative MRI and electron microscopic study. Brain 1991;114(Pt 3):1271-80.
[Google Scholar]
|
| 19. |
Nesbit GM, Forbes GS, Scheithauer BW, Okazaki H, Rodriguez M. Multiple sclerosis: Histopathologic and MR and/or CT correlation in 37 cases at biopsy and three cases at autopsy. Radiology 1991;180:467-74.
[Google Scholar]
|
| 20. |
Downs AM, Lear JT, Bower CP, Kennedy CT. Does influenza vaccination induce bullous pemphigoid? A report of four cases. Br J Dermatol 1998;138:363.
[Google Scholar]
|
| 21. |
Korfitis C, Gregoriou S, Georgala S, Christofidou E, Danopoulou I. Trauma-induced bullous pemphigoid. Indian J Dermatol Venereol Leprol 2009;75:617-9.
[Google Scholar]
|
| 22. |
Bastuji-Garin S, Joly P, Lemordant P, Sparsa A, Bedane C, Delaporte E, et al. Risk factors for bullous pemphigoid in the elderly: A prospective case-control study. J Invest Dermatol 2011;131:637-43.
[Google Scholar]
|
| 23. |
Asbrink E, Hovmark A. Clinical variations in bullous pemphigoid with respect to early symptoms. Acta Derm Venereol 1981;61:417-21.
[Google Scholar]
|
| 24. |
Parker SR, MacKelfresh J. Autoimmune blistering diseases in the elderly. Clin Dermatol 2011;29:69-79.
[Google Scholar]
|
| 25. |
Murrell DF, Daniel BS, Joly P, Borradori L, Amagai M, Hashimoto T, et al. Definitions and outcome measures for bullous pemphigoid: Recommendations by an international panel of experts. J Am Acad Dermatol 2012;66:479-85.
[Google Scholar]
|
| 26. |
Biswas P, Aggarwal I, Sen D, Sumi A, Ghosh A. Bullous pemphigoid clinically presenting as lichen amyloidosis. Indian J Dermatol Venereol Leprol 2014;80:544-6.
[Google Scholar]
|
Fulltext Views
5,670
PDF downloads
3,348





