Translate this page into:
Bullous pemphigoid induced by camrelizumab
Corresponding author: Xiang Nong, Department of Dermatology, First Affiliated Hospital of Kunming Medical University, Kunming, China. nx7011@126.com
-
Received: ,
Accepted: ,
How to cite this article: Wang Q, Tian R, Zhang D, Nong X. Bullous pemphigoid induced by camrelizumab. Indian J Dermatol Venereol Leprol. doi: 10.25259/IJDVL_68_2024
Dear Editor,
A 70-year-old woman, presented with few scattered erythematous plaques and blisters on the trunk with itching and pain. Six months earlier, she was commenced on chemotherapy with camrelizumab (200 mg/3 weeks) for a malignant tumour located in the upper lobe of the left lung. Given the temporal association between the patient’s dermatological symptoms and the initiation of camrelizumab therapy, it was hypothesised that these manifestations might be attributable to the drug. In alignment with the American Society of Clinical Oncology’s recommendations, camrelizumab was temporarily halted. Following this intervention and the application of halometasone, the BP lesions resolved completely. The guidelines further indicate that resumption of immune checkpoint inhibitors (ICIs) is feasible for Grade 2 adverse events (blisters covering 10%–30% of body surface area), provided the lesions are adequately managed.1 Consequently, camrelizumab therapy was reintroduced. However, upon rechallenge, the patient experienced a recurrence and exacerbation of the lesions [Figures 1a and 1b].
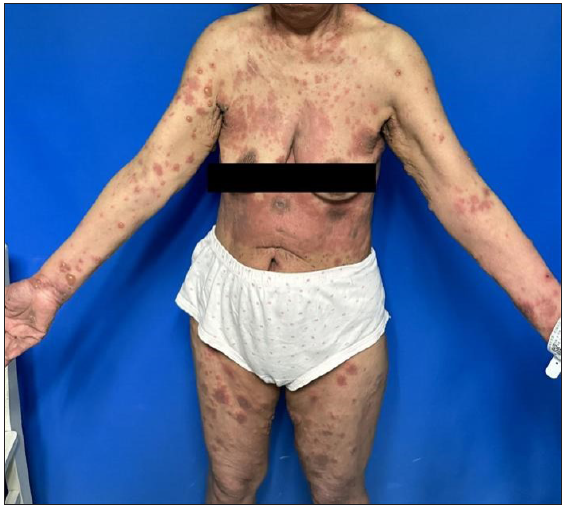
- A 70-year-old female with erythema and blisters scattered on her trunk and limbs.
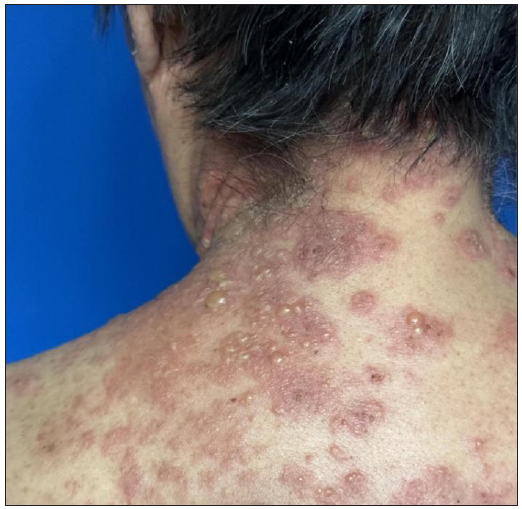
- Tense blisters in the shoulder, Nikolsky sign negative.
Dermatopathological analysis revealed the formation of subepidermal blister accompanied by extensive infiltration of eosinophils, neutrophils and monocytes within the superficial dermis [Figure 2].
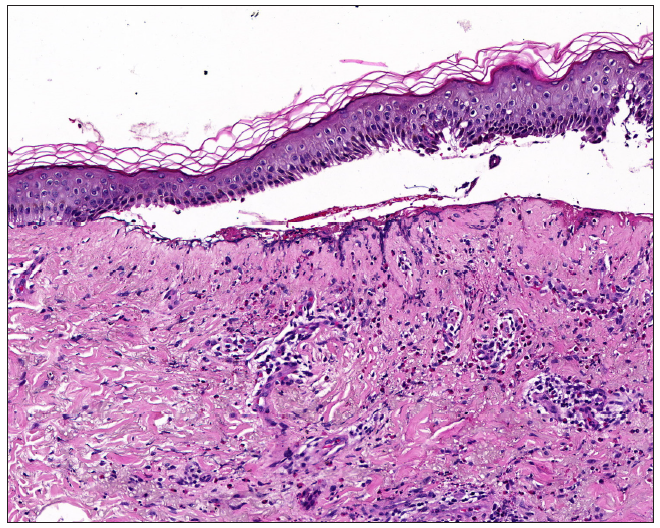
- Subepidermal blister formation, eosinophils, neutrophils, and monocytes infiltration in the superficial dermis (Haematoxylin and eosin, 100x).
Direct immunofluorescence identified the deposition of IgG and C3 along the basement membrane band [Figures 3a and 3b]. Serological testing for Dsg1 and Dsg3 antibodies yielded negative outcomes, whereas the BP180 antibody levels were significantly elevated in serum (134.0 U/mL) and blister fluid (113.8 U/mL). These observations supported the diagnosis of bullous pemphigoid (BP). Methylprednisolone was administered intravenously at a dose of 60 mg daily for the initial three days. Following this period, the dosage was decreased to 40 mg daily for the next three days. It was then further reduced to 32 mg daily for an additional three-day period. Subsequently, the dosage was gradually tapered. The lesions significantly resolved within one month of starting the treatment.
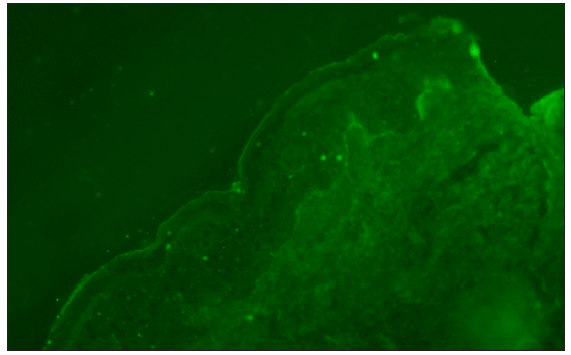
- Linear IgG at basement membrane zone (100x).
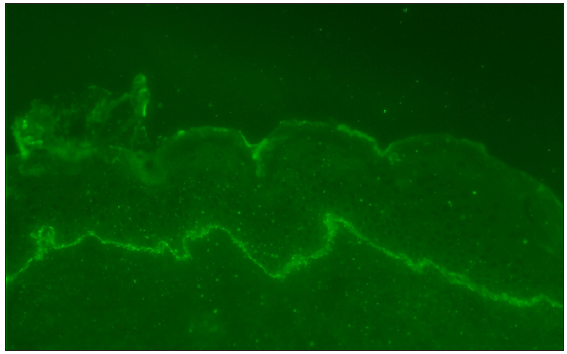
- Linear C3 at basement membrane zone (100x).
Discussion
Bullous pemphigoid (BP) is an autoimmune disease categorised by autoantibodies targeting basement membrane band antigens, including BP180 and BP230 antibodies. DIF shows IgG and C3 deposition along the basement membrane bands.2 The literature reveals a correlation between the incidence of BP and certain medications; notably, new generation of antidiabetic agents, specifically dipeptidyl peptidase 4 inhibitors (DPP4i), as well as immune checkpoint inhibitors that target the programmed cell death protein 1 (PD-1) and its ligand (PD-L1).3
Upregulated PD-L1 expression in malignant cells will inhibit the immunological responses of peripheral T-cells, thereby facilitating tumour evasion from immune surveillance.4 Camrelizumab, a PD-1 inhibitor, is mainly used in treating malignant tumours. A clinical investigation of camrelizumab had documented adverse events, such as reactive cutaneous capillary endothelial proliferation (RCCEP), anaemia and fever, among others.5 To date, there have been no documented cases of BP induced by camrelizumab. Existing literature indicates that BP is the most frequent dermatological adverse effect associated with PD-1 inhibitors, typically emerging 6– 8 months post-therapy initiation. In this context, the patient developed skin lesions 6 months following the initiation of PD-1 inhibitor treatment.6 Furthermore, a recurrence of the lesions was observed upon re-administration of camrelizumab. The correlation between BP and overall malignancy is quivocal, emerging evidence indicates a potential association with hematologic malignancies.2
Following two separate administrations of camrelizumab, the patient exhibited skin lesions on both occasions, which suggests a potential association between BP and the medication. Upon evaluation using the Naranjo Adverse Drug Reaction Probability Scale, which yielded a score of five, the correlation between bullous pemphigoid (BP) and the administration of camrelizumab is categorised as ‘probable’.
The management of BP triggered by PD-1 inhibitors predominantly involves the application of topical glucocorticoids. Additionally, the administration of omalizumab and rituximab has been documented in instances where BP emerged subsequent to the utilisation of PD-1 inhibitors. Although immune checkpoint inhibitors (ICIs), including PD-1/PD-L1 inhibitors, have improved survival rates for patients with malignant tumours, the immunological adverse effects associated with these therapeutics necessitate vigilant clinical oversight.7
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
References
- Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: ASCO guideline update. J Clin Oncol. 2021;39:4073-126.
- [CrossRef] [PubMed] [Google Scholar]
- Association of bullous pemphigoid with malignancy: A systematic review and meta-analysis. J Am Acad Dermatol. 2017;77:691-99.
- [CrossRef] [PubMed] [Google Scholar]
- From molecular insights to clinical perspectives in drug-associated bullous pemphigoid. Int J Mol Sci. 2023;24
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Exploiting CTLA-4, PD-1 and PD-L1 to reactivate the host immune response against cancer. Br J Cancer. 2013;108:1560-5.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Effectiveness and safety of camrelizumab-containing neoadjuvant therapy in patients with esophageal squamous cell carcinoma: A prospective multicenter observational cohort study. J Thorac Dis. 2023;15:6228-37.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- A review of bullous pemphigoid associated with PD-1 and PD-L1 inhibitors. Int J Dermatol. 2018;57:664-69.
- [CrossRef] [PubMed] [Google Scholar]
- Nonbullous pemphigoid secondary to PD-1 inhibition. JAAD Case Rep. 2019;5:898-903.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]





