Translate this page into:
CARD14 associated papulosquamous eruption – A rare cause of erythroderma and successful management with secukinumab
Corresponding author: Dr. Shekhar Neema, Department of Dermatology, Base Hospital, Lucknow, India. shekharadvait@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Gupta A, Neema S, Sandhu S, Mukherjee S, Pathania V. CARD14 associated papulosquamous eruption – A rare cause of erythroderma and successful management with secukinumab. Indian J Dermatol Venereol Leprol. doi: 10.25259/IJDVL_1763_2024
Dear Editor,
Erythroderma is defined as erythema and scaling involving more than 90% of the body surface area.1 Caspase activation and recruitment domain 14 (CARD14) is a gene that activates a protein complex nuclear factor ĸ-B (NF-ĸB), which plays a crucial role in regulating and activating genes involved in inflammatory disorders. In 2018, Craiglow et al. described CARD14-associated papulosquamous eruptions (CAPE) in patients with CARD14 gene mutations. CAPE can present as plaque psoriasis, generalised and localised pustular psoriasis, erythrodermic psoriasis, pityriasis rubra pilaris (PRP), atopic dermatitis, and ichthyosis.2,3 Key features of CAPE include early onset, facial involvement, overlapping features of psoriasis and PRP, and a positive family history. The management of CAPE is challenging due to the lack of established therapeutic guidelines and poor response to conventional systemic agents. Here, we present a case of CAPE that showed an excellent response to secukinumab after failing conventional systemic treatments.
A 2-year-old boy, firstborn child of non-consanguineous parents, presented with generalised redness and scaling for the past 18 months. The child was asymptomatic at birth, but erythema and scaling was noted in the post-auricular area at one month of age. This progressively worsened, covering the entire body over the next five months [Figure 1]. Since then, he was in chronic erythroderma with partial improvement following treatment. It was associated with severe pruritus and multiple hospitalisations. There was no family history of similar complaints. Given the chronic erythroderma, severe pruritus, and spongiotic dermatitis on histopathology, a provisional diagnosis of atopic erythroderma was made. He was intermittently managed with prednisolone (1 mg/kg/day) and cyclosporine (5 mg/kg/day), along with supportive therapy. At three months, there was minimal improvement, leading to the consideration of non-bullous ichthyosiform erythroderma. He was started on acitretin capsules at 1 mg/kg with minimal relief at 3 months. Upon referral to our clinic, his weight was 10.5 kg (25th-50th percentile), and height was 85 cm (75th-90th percentile). He presented with erythroderma with relative sparing of the flexures [Figure 2]. Whole exome sequencing (WES) using next-generation sequencing revealed a heterozygous variant in exon 4 of the CARD14 [c.392C>G (p.Ala131Gly)], consistent with autosomal dominant inheritance. Parental WES revealed the same mutation in the asymptomatic father but not in the mother. He was diagnosed as CAPE. Baseline evaluation was within normal limits, and he was started on secukinumab. The secukinumab dose in the paediatric age group weighing < 50 kg is 75 mg, but a 75 mg vial is unavailable in our country. It has a long- half-life and is safe even in high doses, as seen in dose-ranging studies. Hence, we administered secukinumab 150 mg subcutaneously fortnightly for 2 months, followed by monthly doses. A previous report by Chiramel et al. also suggested that a conventional dosing schedule may not lead to complete clearance, and a higher dosage may be required.4 Remarkable improvement was seen within 10 days of the first dose, and he is in remission for the past year with no treatment-emergent adverse effects [Figure 3a, 3b].
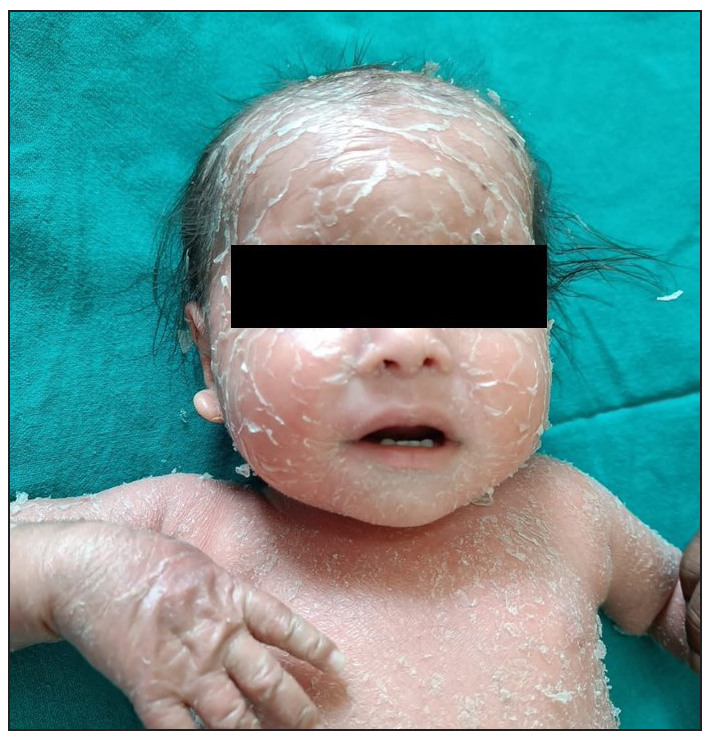
- Patient at 6 months of age with prominent facial involvement.
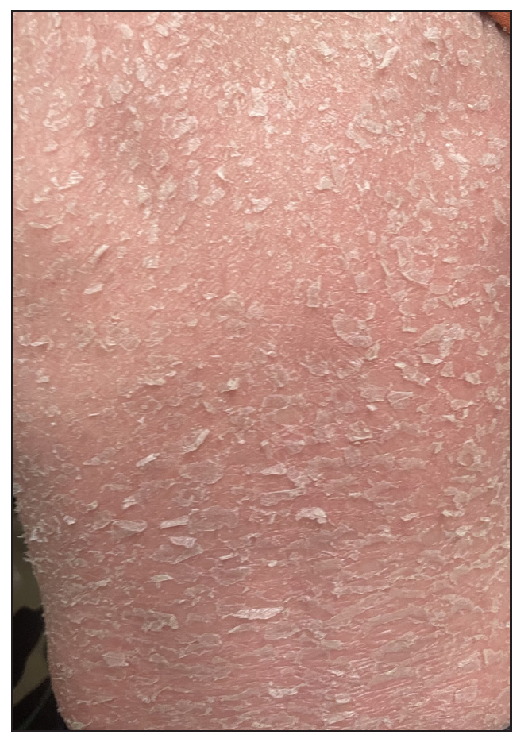
- Erythroderma at 2 years of age with prominent erythema and scaling involving the trunk.
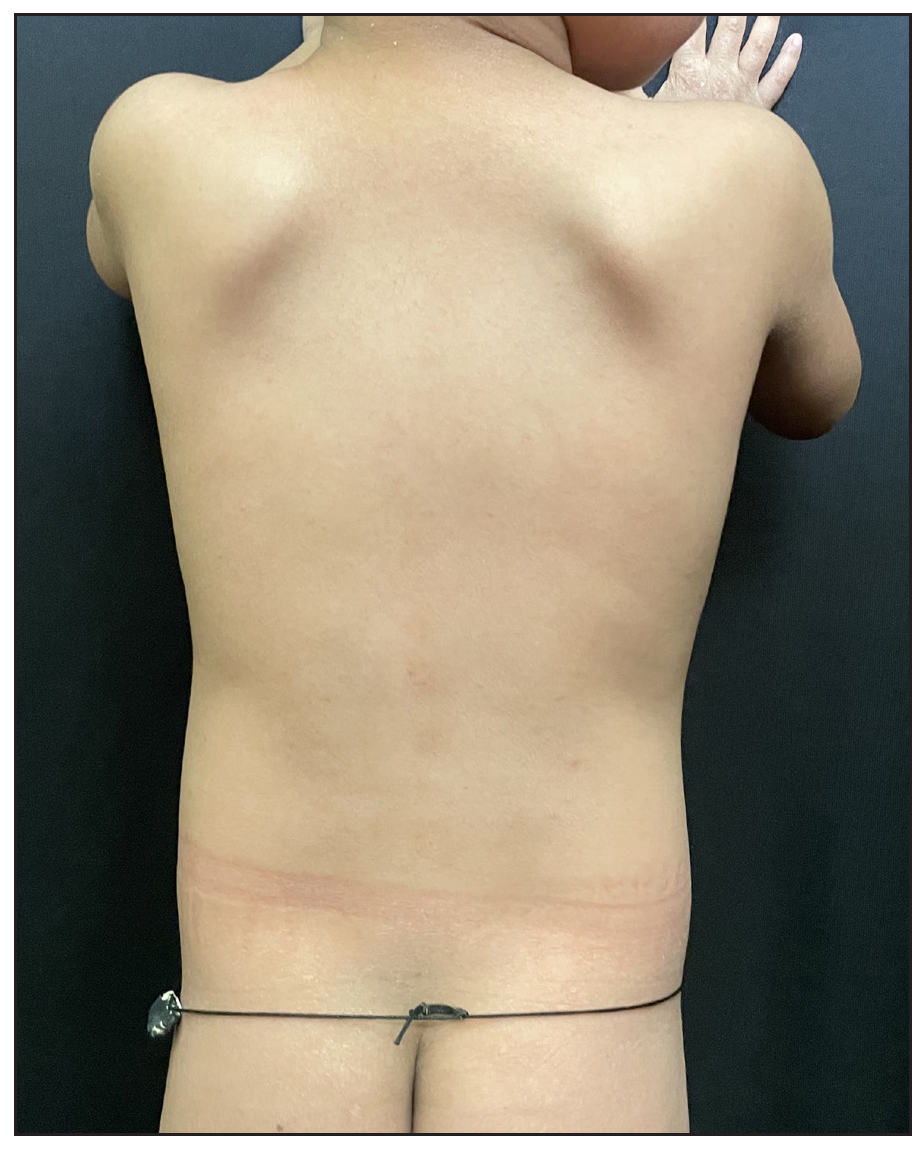
- Complete clearance of skin over the trunk after 12 weeks of secukinumab.
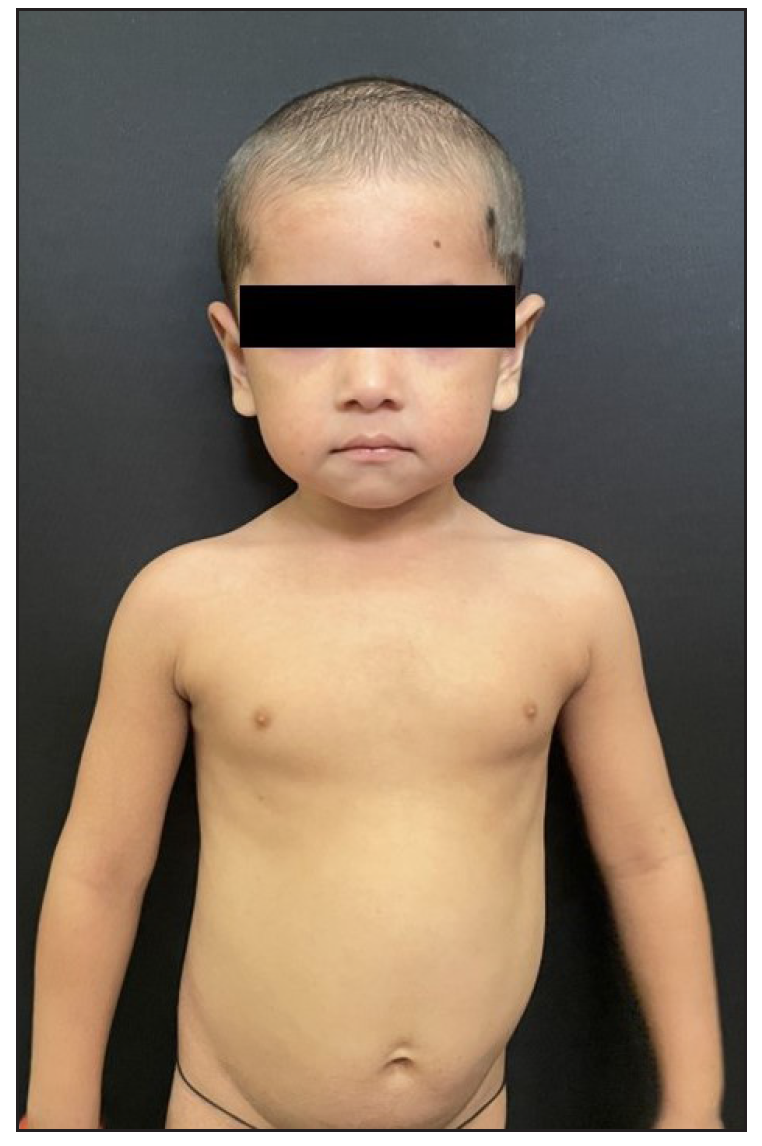
- Face 12 weeks after starting secukinumab.
Gain-of-function mutations in the CARD14 gene have been linked to increased signalling through NF-ĸB. This protein complex plays a vital role in mediating inflammatory reactions in the skin. Increased NF-kB activity leads to elevated levels of chemokines such as IL-8 and CCL-20, which promote the recruitment and differentiation of inflammatory cells. This cascade results in the increased production of pro-inflammatory cytokines like IL-23, IL-17, and IL-22, contributing to dermatological manifestations. The variant seen in our patient is an unreported novel variant. It has been classified as a variant of uncertain significance as per American College of Medical Genetics and Genomics (ACMG) guidelines due to the unavailability of previous reports and the asymptomatic father with the similar variant. However, CAPE is a heterogenous disease and previous studies have reported variants in exons 2,3,4.6,7,9,13,15,18, and 21, with almost 63% reported in exon 3 and 4. The reported variants have incomplete penetrance and have healthy siblings or parents with similar variants.5 In our patient, incomplete penetrance may explain the absence of symptoms in the father. Ustekinumab, an IL-12/23 inhibitor, is particularly effective in managing CAPE.2 Additionally, other biologics such as secukinumab and ixekizumab have been successfully tried.6,7 Due to the unavailability of ustekinumab and the role of IL-17 in CAPE pathogenesis, we opted to use secukinumab. A review of previously published cases managed with biologics are tabulated in the [Supplementary Table 1]S1-S9.
CARD14 gene mutations can be associated with varied clinical manifestations, and diagnostic delays are common, as demonstrated in this case. Dermatologists should maintain a low threshold for genetic testing in patients with overlapping disease features and poor responses to conventional treatments. The administration of biologics can significantly improve the quality of life for these patients.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
References
- The rash that becomes an erythroderma. Clin Dermatol. 2019;37:88-9.
- [CrossRef] [PubMed] [Google Scholar]
- CARD14-associated papulosquamous eruption: A spectrum including features of psoriasis and pityriasis rubra pilaris. J Am Acad Dermatol. 2018;79:487-94.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- A patient with CARD14-associated papulosquamous eruptions showing atopic dermatitis-like features. J Eur Acad Dermatol Venereol. 2021;35:e58-9.
- [CrossRef] [PubMed] [Google Scholar]
- Safety and efficacy of secukinumab in a child with CARD14-associated papulosquamous eruption: A case report and review of literature. Indian Dermatol. Online J. 2024;15:634-6.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical and genetic heterogeneity of CARD14 mutations in psoriatic skin disease. Front Immunol. 2018;9
- [CrossRef] [Google Scholar]
- CARD14-associated papulosquamous eruption flare-up after SARS-CoV-2 infection in a child: Secukinumab a safe and effective treatment. Int J Dermatol. 2023;62:1304-6.
- [CrossRef] [PubMed] [Google Scholar]
- A novel variant with a severe phenotype in a patient with CARD14-associated papulosquamous eruption successfully treated with ixekizumab. Clin Exp Dermatol. 2024;49:661-4.
- [CrossRef] [PubMed] [Google Scholar]






