Translate this page into:
Cardio-pulmonary involvement in systemic sclerosis: A study at a tertiary care center
2 Department of DVL, Gandhi Hospital, Hyderabad, Telangana, India
Correspondence Address:
Geetakiran Arakkal
Department of DVL, Gandhi Medical College and Hospital, Hyderabad, Telangana
India
| How to cite this article: Arakkal G, Chintagunta SR, Chandika V, Damarla SV, Manchala S, Kumar B U. Cardio-pulmonary involvement in systemic sclerosis: A study at a tertiary care center. Indian J Dermatol Venereol Leprol 2017;83:677-682 |
Abstract
Background: Systemic sclerosis is a multisystem disorder characterized by microangiopathy, dysregulation of the immune system and massive deposition of collagen in the connective tissue of the skin, blood vessels and various internal organs. Although the mortality from renal crises has dropped significantly due to the use of angiotensin-converting enzyme inhibitors, cardiac and pulmonary involvement accounts for significant morbidity and mortality. We studied 28 patients with systemic sclerosis at Gandhi Medical College and Hospital, Hyderabad, over a period of two years for cardiopulmonary involvement.Aim: The aim of this study was to analyze the cardiopulmonary involvement in systemic sclerosis.
Methods: All patients with systemic sclerosis attending the dermatology outpatient department were included in the study. The diagnosis of systemic sclerosis was made based on the American Rheumatology Association criteria, and was further confirmed by skin biopsy and serological investigations. X-ray chest, electrocardiogram, two-dimensional ECHO, high-resolution computed tomography chest, pulmonary function tests and bronchoalveolar lavage were done to evaluate cardiopulmonary involvement.
Observations: Out of 28 patients, 17 had diffuse systemic sclerosis and 11 had limited systemic sclerosis. Mean duration of symptoms was 2.9 years. Abnormalities in chest X-ray were found in 16 patients. Pulmonary function tests showed a restrictive pattern in 23 patients. High-resolution computed tomography of the chest showed evidence of interstitial lung disease in 21 patients, while five patients each had pleural effusion and cardiomegaly. Bronchoalveolar lavage showed different cellular patterns such as neutrophilia, eosinophilia and lymphocytosis. Pulmonary arterial hypertension was observed in seven patients and isolated pulmonary arterial hypertension in two patients. Electrocardiogram abnormalities were found in twenty patients. Two-dimensional ECHO was abnormal in 17 patients with valvular abnormalities being the most common finding. Overall, pulmonary involvement was observed in 27 patients and cardiac involvement in 17 patients.
Limitations: A small sample size was a limitation of this study. Diffusing capacity of lung for carbon monoxide, and right cardiac catheterization were not done, as these were not available at our centre.
Conclusions: In our patients, pulmonary involvement was more common than cardiac involvement. Interstitial lung disease and cardiac involvement were more commonly seen in diffuse systemic sclerosis whereas pulmonary hypertension was more frequent in limited systemic sclerosis. Hence, it is important to screen the patients for cardiopulmonary involvement for early diagnosis and treatment and a better prognostic outcome.
Introduction
Systemic sclerosis is a connective tissue disorder characterized by microangiopathy, excess collagen deposition in the skin and dysregulation of the immune system. Although the mortality due to renal involvement has come down, cardiopulmonary manifestations still account for significant morbidity and mortality.
Systemic sclerosis is divided into two groups: Limited and diffuse cutaneous disease. The limited form (limited cutaneous systemic sclerosis) is characterized by thickening of the skin distal to the elbows and knees and is associated with less severe internal organ involvement. The diffuse form (diffuse cutaneous systemic sclerosis) involves skin proximal to the elbows and knees in addition to distal area involvement and is associated with more severe organ damage. Evidence of pulmonary disease is found in over 80% of patients with systemic sclerosis.[1] Pulmonary involvement is second in frequency only to esophageal involvement as a visceral complication of systemic sclerosis and has surpassed renal involvement as the most common cause of death. Interstitial lung disease and pulmonary vascular disease, particularly pulmonary arterial hypertension, are the major types of lung involvement. In combination, they are responsible for 60% of scleroderma-related deaths.[2]
Both interstitial lung disease and pulmonary arterial hypertension may be detected in systemic sclerosis patients with no cardiopulmonary symptoms by using a combination of noninvasive tests for screening, and invasive studies for confirmation. The ability to detect interstitial lung disease and pulmonary arterial hypertension at an early presymptomatic stage, coupled with the availability of pharmacologic interventions may slow the progression of these complications. Cardiac dysfunction is a significant cause of the high morbidity and mortality in systemic sclerosis.[3],[4] The heart involvement in systemic sclerosis is either primary, related to myocardial fibrosis, or secondary, as may occur in cases complicated by pulmonary arterial hypertension or systemic hypertension in those with renal crisis. Cardiac mortality in most studies is attributed to primary cardiac causes (fibrosis) and not to secondary causes.[5] Systemic sclerosis has the highest case-specific mortality; it varies individually depending on the presence and severity of organ involvement, systemic sclerosis subsets, age at diagnosis and gender differences. Although not curable, there have been substantial advances in treatment options for organ-based complications of systemic sclerosis. Early diagnosis and individually tailored therapy help to manage this disorder.
Methods
The present study was conducted from October 2012 to September 2014 at Gandhi Hospital, Hyderabad, Telangana. It was a prospective observational study of 28 patients with clinical manifestations of systemic sclerosis irrespective of age, sex and duration of disease. Pregnant women, patients with overlap syndromes and patients with cardiac or pulmonary abnormalities prior to the onset of systemic sclerosis were excluded from the study. A detailed history was obtained and general, cutaneous and systemic clinical evaluation was done. The diagnosis of systemic sclerosis was made based on the American Rheumatology Association criteria,[6] and was further confirmed by skin biopsy and serological investigations such as antinuclear antibody, antiScl 70 antibodies and anticentromere antibodies. Routine investigations such as complete blood picture, erythrocyte sedimentation rate, complete urine examination, 24 h urine protein, random blood sugar, renal function test, liver function test and serum electrolytes were done. Specific investigations including X-ray chest, electrocardiogram, two-dimensional ECHO, high-resolution computed tomography chest, pulmonary function tests and bronchoalveolar lavage were conducted to evaluate cardiopulmonary involvement. Ultrasonography abdomen and barium swallow/endoscopy were done to detect involvement of other systems. Pulmonary function tests were assessed as follows: If forced vital capacity was <70% of the predicted value and forced expiratory volume in 1 s/forced vital capacity ratio was >80% of the predicted value, restrictive pattern was considered. Restrictive pattern was graded as mild, moderate and severe, if forced expiratory volume in 1 s was 60–79%, 40–59% and <40%, respectively. Interstitial lung disease was diagnosed based on high-resolution computed tomography findings such as ground glass opacities, architectural distortion, honey combing, septal thickening, reticulations and tractional brochiolectasis. Pulmonary arterial hypertension, left ventricular diastolic dysfunction and right ventricular hypertrophy were diagnosed based on two-dimensional ECHO findings. Pulmonary hypertension, defined by a mean arterial pressure ≥25 mmHg at rest, was usually confirmed by right heart catheterization.[7] Pulmonary arterial hypertension was graded as mild, moderate and severe, if right ventricular systolic pressure was 30–40 mm of Hg, 40–60 mm of Hg and >60 mm of Hg, respectively. Diastolic dysfunction refers to abnormality of heart muscle relaxation during diastole, E < A and high left ventricular end diastolic pressure (E - early filling velocity of left ventricle, A - late filling velocity of left ventricle (atrial kick). E > A is the normal pattern.) suggest left ventricular diastolic dysfunction.[8] Right ventricular hypertrophy was defined as right ventricular wall thickening due to chronic pressure overload, seen on ECHO as greater than 9 mm thickness.
Observations and Results
Out of 28 patients, 27 were females and one patient was male with a ratio of 27:1. Based on the extent of skin involvement, patients were classified as diffuse cutaneous systemic sclerosis 17 (60.7%) and limited cutaneous systemic sclerosis 11 (39.3%). The duration of symptoms varied from 3 months to 7 years. The age ranged between 11 and 60 years (mean = 36.7 years). The most common cutaneous features observed were tightening of skin (100%), Raynaud's phenomenon (85.7%) and pigmentary changes (82%).
Cardiopulmonary symptoms such as dyspnea (64.3%), cough (32%), palpitations (14.3%) and chest pain (10.7%) were reported in 18 (64.3%) out of 28 patients. The mean duration of symptoms in diffuse cutaneous systemic sclerosis was 2.2 years and 4 years in limited cutaneous systemic sclerosis. Out of 28 patients, clinical signs of cardiopulmonary involvement were noted in 71.4%. There was decreased chest expansion in 5, crepitations in 11, rhonchi in 2 and loud P2 in 6 patients.
Routine investigations revealed a raised erythrocyte sedimentation rate in 53.6%, hemoglobin less than 10 g% in 42.9%, micro albuminuria in 35.7% and macro albuminuria in 17.9% patients. Skin biopsy was done in all cases and was consistent with the clinical diagnosis. Antinuclear antibody was positive in 23 (82%) patients. Speckled pattern was the most common finding in 17 (73.9%) followed by homogenous in 5 (21.7%) patients and granular in 1 (4.4%) patient. Antiscl-70 antibodies were the most common, seen in 50 per cent. Antiscl-70 antibodies were observed in 11 (78.6%) patients of diffuse cutaneous systemic sclerosis and 3 (21.4%) patients of limited cutaneous systemic sclerosis. Anti-centromere antibody was positive in 4 (14.2%) patients, all of who had limited cutaneous systemic sclerosis.
On further investigation, cardiopulmonary involvement was observed in 27 (96.4%) patients, out of which, 17 (60.7%) had both pulmonary and cardiac manifestations and 10 (35.7%) had pulmonary manifestations alone. Pulmonary involvement was seen in 16 (59.3%) cases of diffuse cutaneous systemic sclerosis and in 11 (40.7%) cases of limited cutaneous systemic sclerosis.
Abnormal chest X-ray findings were seen in 16 (57.2%) patients. Prominent bronchovascular markings were noted in 7 (25%) patients, mild cardiomegaly in 5 (17.9%) and bilateral reticulonodular opacities, bilateral apical healed calcific lesions, small focal bronchiectatic area in right lung and pleural effusion in 1 (3.6%) patient each.
Pulmonary function test showed abnormalities in 26 (92.9%) patients. Moderate restrictive pattern was observed in 12 (52.2%) patients, severe restrictive in 7 (30.4%) and mild restrictive pattern in 4 (17.4%) patients. Restrictive pattern was found in 15 (88.2%) out of 17 patients with diffuse cutaneous systemic sclerosis patients, compared to 8 (72.7%) out of 11 patients with limited cutaneous systemic sclerosis.
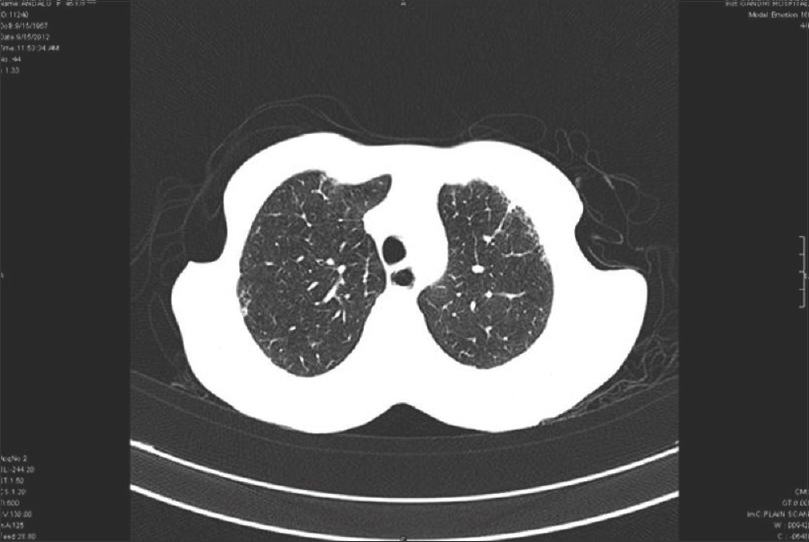
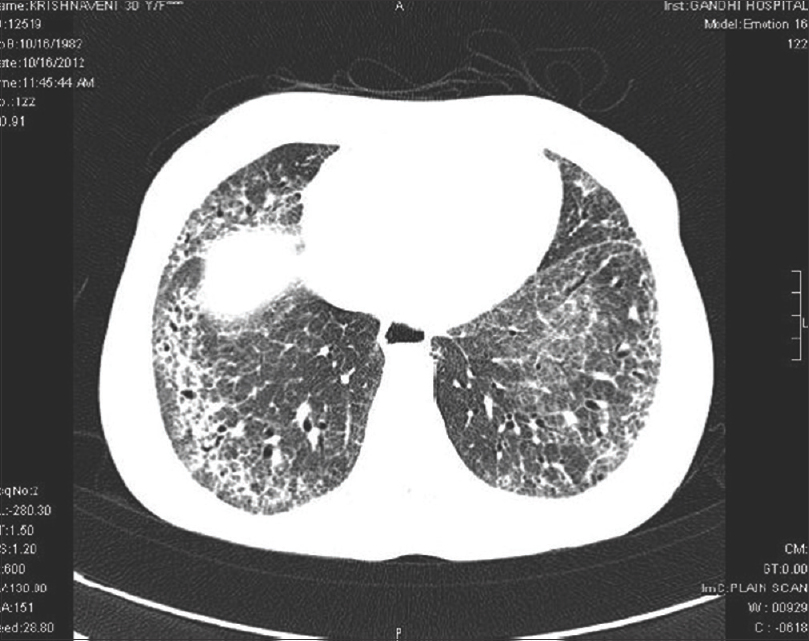
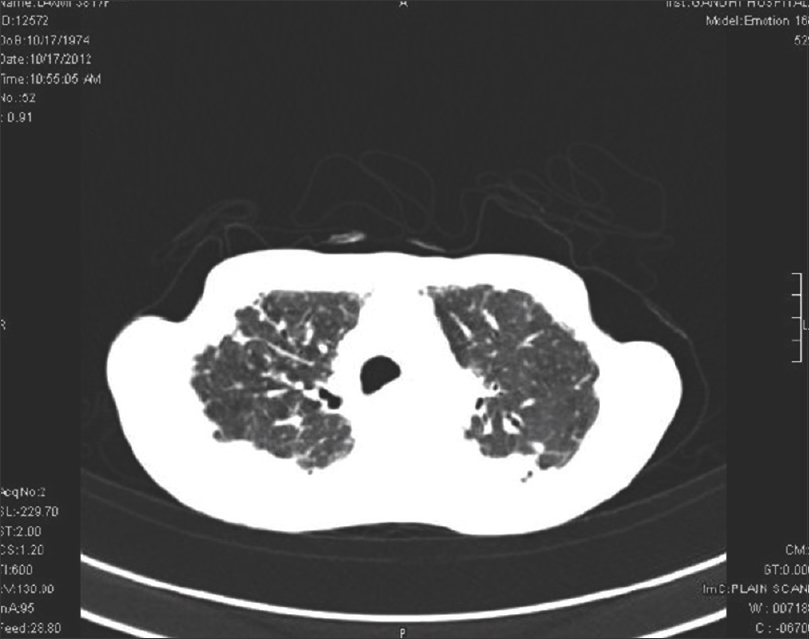
High-resolution computed tomography chest showed interstitial lung disease in 14 (66.7%) patients of diffuse cutaneous systemic sclerosis and 7 (33.3%) of limited cutaneous systemic sclerosis. Interstitial lung disease was the most common feature observed in 21 (75%) patients, dilated esophagus was seen in 7 (25%), and pleural effusion and cardiomegaly in 5 (17.9%) patients each. Ground glass opacities were the most common finding in 14 (66.7%), honeycombing in 12 (57%), architectural distortion in 10 (47.6%), septal thickening in 10 (47.6%), reticulations in 9 (42.9%) and tractional bronchiectasis in 5 (23.8%) patients [Figure - 1], [Figure - 2], [Figure - 3], [Figure - 4].
 |
| Figure 1: High-resolution computed tomography chest showing patchy areas of honey combing peripherally distributed in the posterior segments of lower lobes |
 |
| Figure 2: High-resolution computed tomography chest – patchy areas of early honey combing in both upper lobes and dilated esophagus |
 |
| Figure 3: High-resolution computed tomography – extensive reticulonodular opacities and honey combing in both upper and lower lobes. Tractional bronchiolectasis in the right lower lobe |
 |
| Figure 4: High-resolution computed tomography – distortion of parenchymal architecture with peribroncho-vascular interstitial septal thickening |
Bronchoalveolar lavage was done in 21 patients with interstitial lung disease as detected on high-resolution computed tomography of the chest. Neutrophilia was the common cellular pattern observed in 12 (57.1%), eosinophilia in 8 (38.1%) and lymphocytosis in 4 (19%) patients.
Electrocardiogram findings were abnormal in 20 (71.4%) patients. Right axis deviation was found in 7 (25%), nonspecific ST-T wave changes and sinus tachycardia in 5 (17.9%) each and sinus bradycardia in 3 (10.7%) patients.
Out of 17 patients having two-dimensional ECHO abnormalities, 10 (58.8%) had diffuse cutaneous systemic sclerosis and 7 (41.2%) had limited cutaneous systemic sclerosis. Valvular abnormalities were the most common findings observed in 14 (50%) patients. Right ventricular hypertrophy was seen in 7 (25%) and left ventricular diastolic dysfunction in 7 (25%) patients. Decreased left ventricular ejection fraction was a feature in 5 (17.9%) patients, tachycardia in 5 (17.9%) and minimal pericardial effusion was present in 1 (3.6%) patient.
Mild tricuspid regurgitation was observed in 8 (57.2%) patients followed by mild mitral regurgitation in 3 (21.4%), mild aortic regurgitation in 1 (7.2%), anterior mitral leaflet prolapse in 1 (7.2%) and sclerotic aortic valve in one (7.2%) patient.
Pulmonary arterial hypertension was detected by two-dimensional ECHO in 7 (25%) patients as mild in 5 (71.4) moderate in 1 (14.3) and severe in 1 (14.9). Out of 7 patients, 5 (71.4%) were of limited cutaneous systemic sclerosis and 2 (28.6%) were of diffuse cutaneous systemic sclerosis. Isolated pulmonary arterial hypertension was observed in 2 (18.2%) out of 11 patients of limited cutaneous systemic sclerosis, which was not observed in diffuse cutaneous systemic sclerosis. Pulmonary arterial hypertension associated with interstitial lung disease was observed in 3 (60%) cases of limited cutaneous systemic sclerosis, and 2 (40%) of diffuse cutaneous systemic sclerosis.
Discussion
Cardiopulmonary symptoms were reported in 18 (64.3%) out of 28 patients, and clinical signs were present in 20 (71.4%) patients. On specific investigation, cardiopulmonary involvement was detected in 27 (96.4%), out of which 17 (60.7%) had both pulmonary and cardiac manifestations and 10 (35.7%) had pulmonary manifestations alone.
In our study, abnormal chest X-ray findings were observed in 16 (57.1%) patients whereas Sharma et al. observed it in 65.3% in their study.[9]
In the present study, pulmonary function tests were abnormal in 26 (92.9%) and 23 (82.1%) presented with restrictive pattern. However, in the study of Sharma et al., 85.8% patients had abnormal pulmonary function tests. Steen et al. noted a restrictive pattern in observed 40% of patients of who 67.7% had moderate and 32.3% had severe restrictive patterns.[10] In the present study, restrictive pattern was observed in 15 (88.2%) patients with diffuse systemic sclerosis and 8 (72.7%) with limited cutaneous systemic sclerosis, whereas Kane et al. observed a restrictive pattern in 50% and 30% of patients with diffuse systemic sclerosis and limited systemic sclerosis, respectively.[11]
In our study, high-resolution computed tomography chest showed features suggestive of interstitial lung disease in 21 (75%) with ground glass opacities in 14 (66.7%), honeycombing in 12 (57.1%) and reticulations in 9 (42.9%) patients. However, in the study of Goldin et al., ground glass opacities and honeycombing were observed in 49.4% and 37.2% patients, respectively.[12] Wells et al. observed a reticular pattern in 51.9% patients.[13]
In the present study, interstitial lung disease was observed in 21 (75%) patients. Of them, 14 (66.7%) had diffuse cutaneous systemic sclerosis and 7 (33.3%) had limited cutaneous systemic sclerosis, whereas in the study of Perera et al., interstitial lung disease was observed in 53.8%. Of them, 78.9% had diffuse cutaneous systemic sclerosis and 21.1% had limited cutaneous systemic sclerosis.
Bronchoalveolar lavage revealed different cellular patterns showing neutrophilia in 12 (57.1%), eosinophilia in 8 (38.1%) and lymphocytosis in 4 (19.1%) patients. However, in the study done by Goh et al., neutrophilia, eosinophilia and lymphocytosis were observed in 47%, (43%) and (26%) patients, respectively.[14]
In the present study, pulmonary arterial hypertension detected by ECHO was observed in 7 (25%) patients. Of them, 5 (71.4%) had limited systemic sclerosis and 2 (28.6%) had systemic sclerosis. However, Morelli et al. observed pulmonary arterial hypertention in 51.9% patients.[15] Of them, 55.6% had limited cutaneous systemic sclerosis and 44.4% had diffuse cutaneous systemic sclerosis. Isolated pulmonary arterial hypertension was observed in 2 (18.2%) patients of 11 patients with limited cutaneous systemic sclerosis and was not observed in diffuse cutaneous systemic sclerosis in the present study, whereas in the study of Sacks et al., isolated pulmonary arterial hypertention was found in 10% and 2% of patients with limited cutaneous systemic sclerosis and diffuse cutaneous systemic sclerosis, respectively.[16]
Pulmonary arterial hypertension associated with interstitial lung disease was observed in 5 (17.9%) patients in the present study, similar to the study done by Chang et al. whereas the prevalence of combined pulmonary arterial hypertension and interstitial lung disease ranged between 18% and 22%.[17]
In our study, pulmonary involvement was observed in 27 (96.4%) patients, of them 16 (59.3%) had diffuse cutaneous systemic sclerosis and 11 (40.7%) had limited cutaneous systemic sclerosis, whereas in the study of Perera et al., pulmonary involvement was observed in 72.6% of patients, of them 81.2% and 18.8% patients had diffuse cutaneous systemic sclerosis and limited cutaneous systemic sclerosis, respectively.[18]
In the present study, electrocardiogram was abnormal in 20 (71.4%) patients with non-specific ST-T wave changes in 5 (17.9%), right axis deviation in 7 (25%), sinus tachycardia in 5 (17.9%) and sinus bradycardia in 3 (10.7%) patients. However, in the study of Draeger et al., electrocardiogram was abnormal in 140 (52.8%) patients with nonspecific ST-T wave changes in 32 (12.1%), right axis deviation in 13 (4.9%), sinus tachycardia in 7 (2.6%) and sinus bradycardia in 19 (7.2%) patients.[19]
In the present study, two-dimensional ECHO was abnormal in 17 (60.7%) patients. Of them, 10 (58.8%) patients had diffuse cutaneous systemic sclerosis and 7 (41.2%) had limited cutaneous systemic sclerosis, whereas in the study by Thompson and Pope, two-dimensional ECHO was abnormal in 64.9% patients.[20] Of them, 41.7% and 58.3% had diffuse cutaneous systemic sclerosis and limited cutaneous systemic sclerosis, respectively.
In the present study, the left ventricular ejection fraction was decreased in 5 (17.9%) patients whereas a study by Allanore et al. showed decreased left ventricular ejection fraction in 5.4% patients.[21] Hypokinesia of the left ventricle was observed in one (3.6%) patient whereas in the study of Aguglia et al., it was observed in 29% patients.[22] Left ventricular diastolic dysfunction was observed in 7 (25%) patients, however Aguglia et al. reported this abnormality in 62.1% in their study.[23]
Pericardial effusion was found in 1 (3.6%) patient in the present study, whereas a study by Thompson and Pope reported it in 5.4%.
The most common lung manifestations in systemic sclerosis are interstitial lung disease followed by pulmonary arterial hypertension, which are responsible for significant morbidity and mortality. Interstitial lung disease accounts for 40% of cases of lung involvement in diffuse cutaneous systemic sclerosis. Cardiopulmonary symptoms and signs were reported in 64.3% and 71.4% of our patients, respectively, while on specific investigation, cardiopulmonary involvement was detected in 96.4 per cent There was no correlation between the severity of skin involvement and pulmonary manifestations. Patients may remain asymptomatic despite the presence of clinical signs such as crackling on auscultation, and interstitial thickening on X-ray. Pulmonary function test abnormalities may precede symptoms, or changes in the chest X-ray. Normal spirometry cannot exclude early interstitial lung disease changes. A decrease in diffusing capacity of lung for carbon monoxide correlates with the severity of interstitial lung disease detected by high-resolution computed tomography.
Pulmonary arterial hypertension may occur as an isolated feature or in association with interstitial lung disease. The prevalence of pulmonary arterial hypertension detected by echocardiogram varies from 13–35% to 7–13% with right cardiac catheterization.[24],[25],[26],[27] Our study also showed pulmonary arterial hypertension in 25% of patients by ECHO.
Cardiac involvement in systemic sclerosis indicates a poor prognosis. Clinically, it is difficult to detect the early changes of cardiac involvement. Studies have shown that there is a gross disparity in cardiac involvement between clinical and autopsy findings.[24],[28],[29],[30]
Limitations
The limitation of this study is a small sample size due to limited resources and time. Follow-up investigations were not done.
Diffusing capacity of lung for carbon monoxide is an important prognostic indicator of lung pathology that could not be done due to its non-availability at our centre. Right cardiac catheterization also could not be performed.
Conclusions
Patients with systemic sclerosis may present with systemic complications such as pulmonary arterial hypertension and interstitial lung disease during the course of the disease; signs and symptoms do not always correlate with the type and duration disease. Chest X-ray is a useful screening tool, but is not verysensitive for the detection of interstitial lung disease. Impaired pulmonary function tests (forced vital capacity and diffusing capacity of lung for carbon monoxide) are a poor prognostic marker of lung involvement. High-resolution computed tomography has a high diagnostic sensitivity, but abnormalities are difficult to interpret. Thorough evaluation in cases of systemic sclerosis is essential, with an integration of symptoms and specific investigations (pulmonary function test, ECHO and high-resolution computed tomography) for early diagnosis, treatment and better prognostic outcome.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Rodnan GP, Benedek TG. An historical account of the study of progressive systemic sclerosis (diffuse scleroderma). Ann Intern Med 1962;57:305-19.
[Google Scholar]
|
| 2. |
Barnett AJ. History of scleroderma. In: Clements PJ, Furst DE, editors. Systemic Sclerosis. Baltimore: Williams and Wilkins; 1996. p. 3-22.
[Google Scholar]
|
| 3. |
Ioannidis JP, Vlachoyiannopoulos PG, Haidich AB, Medsger TA Jr., Lucas M, Michet CJ, et al. Mortality in systemic sclerosis: An international meta-analysis of individual patient data. Am J Med 2005;118:2-10.
[Google Scholar]
|
| 4. |
Steen VD, Medsger TA. Changes in causes of death in systemic sclerosis, 1972-2002. Ann Rheum Dis 2007;66:940-4.
[Google Scholar]
|
| 5. |
Osler W. The Principles and Practice of Medicine. New York: Appleton; 1894. p. 993.
[Google Scholar]
|
| 6. |
Van den Hoogen, Khanna D, Fransen J, Johnson SR, Baron M, Tyndall A, et al. Classification criteria for systemic sclerosis. Arthritis and Rheumatism.2013;65:2737-47.
[Google Scholar]
|
| 7. |
Hoeper MM, Bogaard HJ, Condliffe R, Frantz R, Khanna D, Kurzyna M, et al. Definitions and diagnosis of pulmonary hypertension. J Am Coll Cardiol 2013;62 25 Suppl:D42-50.
[Google Scholar]
|
| 8. |
Galderisi M. Diastolic dysfunction and diastolic heart failure: Diagnostic, prognostic and therapeutic aspects. Cardiovasc Ultrasound 2005;3:9.
[Google Scholar]
|
| 9. |
Sharma VK, Trilokraj T, Khaitan BK, Krishna SM. Profile of systemic sclerosis in a tertiary care center in North India. Indian J Dermatol Venereol Leprol 2006;72:416-20.
[Google Scholar]
|
| 10. |
Steen VD, Conte C, Owens GR, Medsger TA Jr. Severe restrictive lung disease in systemic sclerosis. Arthritis Rheum 1994;37:1283-9.
[Google Scholar]
|
| 11. |
Kane GC, Varga J, Conant EF, Spirn PW, Jimenez S, Fish JE. Lung involvement in systemic sclerosis (scleroderma): Relation to classification based on extent of skin involvement or autoantibody status. Respir Med 1996;90:223-30.
[Google Scholar]
|
| 12. |
Goldin JG, Lynch DA, Strollo DC, Suh RD, Schraufnagel DE, Clements PJ, et al. High-resolution CT scan findings in patients with symptomatic scleroderma-related interstitial lung disease. Chest 2008;134:358-67.
[Google Scholar]
|
| 13. |
Wells AU, Hansell DM, Rubens MB, Cullinan P, Haslam PL, Black CM, et al. Fibrosing alveolitis in systemic sclerosis. Bronchoalveolar lavage findings in relation to computed tomographic appearance. Am J Respir Crit Care Med 1994;150:462-8.
[Google Scholar]
|
| 14. |
Goh NS, Veeraraghavan S, Desai SR, Cramer D, Hansell DM, Denton CP, et al. Bronchoalveolar lavage cellular profiles in patients with systemic sclerosis-associated interstitial lung disease are not predictive of disease progression. Arthritis Rheum 2007;56:2005-12.
[Google Scholar]
|
| 15. |
Morelli S, Barbieri C, Sgreccia A, Ferrante L, Pittoni V, Conti F, et al. Relationship between cutaneous and pulmonary involvement in systemic sclerosis. J Rheumatol 1997;24:81-5.
[Google Scholar]
|
| 16. |
Sacks DG, Okano Y, Steen VD, Curtiss E, Shapiro LS, Medsger TA Jr. Isolated pulmonary hypertension in systemic sclerosis with diffuse cutaneous involvement: Association with serum anti-U3RNP antibody. J Rheumatol 1996;23:639-42.
[Google Scholar]
|
| 17. |
Chang B, Wigley FM, White B, Wise RA. Scleroderma patients with combined pulmonary hypertension and interstitial lung disease. J Rheumatol 2003;30:2398-405.
[Google Scholar]
|
| 18. |
Perera A, Fertig N, Lucas M, Rodriguez-Reyna TS, Hu P, Steen VD, et al. Clinical subsets, skin thickness progression rate, and serum antibody levels in systemic sclerosis patients with anti-topoisomerase I antibody. Arthritis Rheum 2007;56:2740-6.
[Google Scholar]
|
| 19. |
Draeger DT, Assassi S, Sharif R, Gonzalez EB, Harper BE, Arnett FC, et al. Right bundle branch block: A predictor of mortality in early systemic sclerosis. PLoS One 2014;9:10.
[Google Scholar]
|
| 20. |
Thompson AE, Pope JE. A study of the frequency of pericardial and pleural effusions in scleroderma. Br J Rheumatol 1998;37:1320-3.
[Google Scholar]
|
| 21. |
Allanore Y, Meune C, Vonk MC, Airo P, Hachulla E, Caramaschi P, et al. Prevalence and factors associated with left ventricular dysfunction in the EULAR Scleroderma Trial and research group (EUSTAR) database of patients with systemic sclerosis. Ann Rheum Dis 2010;69:218-21.
[Google Scholar]
|
| 22. |
Hegedüs I, Czirják L. Left ventricular wall motion abnormalities in 80 patients with systemic sclerosis. Clin Rheumatol 1995;14:161-4.
[Google Scholar]
|
| 23. |
Aguglia G, Sgreccia A, Bernardo ML, Carmenini E, Giusti De Marle M, Reali A, et al. Left ventricular diastolic function in systemic sclerosis. J Rheumatol 2001;28:1563-7.
[Google Scholar]
|
| 24. |
D'Angelo WA, Fries JF, Masi AT, Shulman LE. Pathologic observations in systemic sclerosis (scleroderma). A study of fifty-eight autopsy cases and fifty-eight matched controls. Am J Med 1969;46:428-40.
[Google Scholar]
|
| 25. |
Weaver AL, Divertie MB, Titus JL. Pulmonary scleroderma. Dis Chest 1968;54:490-8.
[Google Scholar]
|
| 26. |
Schurawitzki H, Stiglbauer R, Graninger W, Herold C, Pölzleitner D, Burghuber OC, et al. Interstitial lung disease in progressive systemic sclerosis: High-resolution CT versus radiography. Radiology 1990;176:755-9.
[Google Scholar]
|
| 27. |
Avouac J, Airò P, Meune C, Beretta L, Dieude P, Caramaschi P, et al. Prevalence of pulmonary hypertension in systemic sclerosis in European Caucasians and metaanalysis of 5 studies. J Rheumatol 2010;37:2290-8.
[Google Scholar]
|
| 28. |
Medsger TA Jr., Masi AT, Rodnan GP, Benedek TG, Robinson H. Survival with systemic sclerosis (scleroderma). A life-table analysis of clinical and demographic factors in 309 patients. Ann Intern Med 1971;75:369-76.
[Google Scholar]
|
| 29. |
Sackner MA, Akgun N, Kimbel P, Lewis DH. The pathophysiology of scleroderma involving the heart and respiratory system. Ann Intern Med 1964;60:611-30.
[Google Scholar]
|
| 30. |
Bulkley BH, Ridolfi RL, Salyer WR, Hutchins GM. Myocardial lesions of progressive systemic sclerosis. A cause of cardiac dysfunction. Circulation 1976;53:483-90.
[Google Scholar]
|
Fulltext Views
3,637
PDF downloads
1,226





