Translate this page into:
Cardiovascular risk markers in premature canities
Corresponding Author: Dr. Suparna Das, Department of Dermatology and STD, Lady Hardinge Medical College, Connaught Place, New Delhi, Delhi, 110001, India. rinku.ria88@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Das S, Chander R, Garg T, Mendiratta V, Singh R, Sanke S. Cardiovascular risk markers in premature canities. Indian J Dermatol Venereol Leprol 2023;89:221-5.
Abstract
Background:
An elevated cardiovascular risk has been demonstrated in middle-aged individuals with onset of hair greying before the age of 30 years. Increased serum levels of pro-inflammatory cytokines, interleukin-6 (IL-6) and tumour necrosis factor-alpha (TNF-α), indicate an ongoing state of chronic inflammation that is correlated with cardiovascular risk but have not been studied earlier in patients with early onset of hair greying.
Aim/Objective:
To study various cardiovascular risk markers including pro-inflammatory cytokines interleukin-6 (IL-6) and tumour necrosis factor-alpha (TNF-α) in patients with premature canities.
Methods:
This was a hospital-based case-control study of 40 patients with premature canities (age between 19 and 25 years; >5 grey hair) and an equal number of age and gender-matched healthy controls. The blood pressure, pulse rate and body mass index were recorded, and investigations including fasting blood sugar, serum insulin, fasting lipid profile, high sensitivity c-reactive protein (hs-CRP), IL-6 and TNF-α were performed. The homeostatic model assessment of insulin resistance (HOMA-IR) was calculated for all the participants.
Results:
The mean blood pressure, fasting blood sugar, serum insulin, hs-CRP and HOMA-IR were all significantly elevated in patients with premature canities and the serum HDL levels were significantly lower. A greater number of patients with premature canities had significantly elevated IL-6 as compared with the controls.
Limitations:
The sample size was small. A subjective scale was used for grading the severity of premature canities. Trichoscopic evaluation of severity of greying or modified phototrichogram could not be used in this study.
Conclusion:
Abnormalities in cardiovascular risk markers were found in patients with premature canities. Screening and counselling of patients with premature greying of hair is recommended in order to prevent future cardiovascular disease.
Keywords
Atherosclerosis
cardiovascular
premature canities
Plain Language Summary
This study was conducted to demonstrate the cardiovascular risk in patients of premature canities. These patients were found to have raised blood glucose, lipids, high sensitivity c-reactive protein, pro-inflammatory cytokine levels and hypertension and metabolic syndrome which are all demonstrative of increased cardiovascular risk in this patient population. Thus, identifying these cases with increased cardiovascular risk and subjecting them to timely therapies may lead to reduced burden of adverse cardiovascular events in the future.
Introduction
Premature canities is the onset of greying of hair before the age of 20 years in Caucasians, 25 years in Asians and 30 years in Africans.1 The underlying defect in canities is a depletion of the melanocyte stem cell pool.2 A decline in the cell replicating potential with advancing age results in programmed cell death. Increasing oxidative stress, psychological stress and inflammation cause defective migration and recruitment of melanocytes leading to senile canities.1,3-9
Increased levels of pro-oxidants and inflammatory cytokines [e.g., interleukin-6 (IL-6) and tumour necrosis factor-alpha (TNF-α)] and decreased levels of antioxidants cause apoptosis of melanocyte stem cells resulting in canities.1,3-9 The presence of cardiovascular risk factors result in a pro-inflammatory background favouring depletion of the melanocyte stem cell pool, thus accelerating the onset of canities.3
Although there have been reports of the presence of cardiovascular risk factors in young individuals (<25 years) with early onset canities, serum levels of pro-inflammatory cytokines (IL-6 and TNF-α) have not been studied. In this study we assessed cardiovascular risk in patients with premature greying.
Materials and Methods
This hospital-based case-control study was conducted from November 2018 to April 2020 at the dermatology outpatient department of Smt. Sucheta Kriplani Hospital, a tertiary care centre. The study was approved by the Institutional Ethics Committee. Forty patients with premature canities between the ages of 19 and 25 years with >5 grey hair and age and sex- matched healthy controls were recruited from the Dermatology OPD. Patients were excluded from the study if they
had a history of hypothyroidism, psoriasis or vitiligo
had taken chloroquine, epidermal growth factor receptor inhibitors, mephenesin, phenylthiourea, triparanol, fluoro-butyrophenones or interferon-alpha (IFN-α) in the last six months, or
had previously used topical application of dyes, dithranol, chrysarobin, resorcin or prostaglandin (PG)-F2α analogues in past three months.
A detailed history and examination including height, weight, waist circumference, blood pressure and pulse rate were recorded for all the participants. A 10 mL venous sample for lipid profile, fasting blood sugar, serum insulin, IL-6, TNF-α and high sensitivity c-reactive protein (hs-CRP) was then taken. The homeostatic model assessment of insulin resistance (HOMA-IR) index was calculated from fasting blood sugar and serum insulin.
Greying severity assessment was performed by grasping approximately 50–60 hair between two fingers near the scalp surface on five areas of the scalp (frontal, parietal, bitemporal and occipital regions) and the number of grey hair was counted in each of the five regions. Patients were classified as having mild (<25 grey hair), moderate (25–50 grey hair), or severe (>50 grey hair) canities.
SPSS software version 23 was used for statistical analysis. Continuous variables were expressed as mean ± standard deviation and were analysed using the t-test. Categorical variables were expressed as frequencies and percentages and analysed using the Chi-square test. Statistical significance was assumed at a P-value of <0.05.
Results
Patient characteristics [Table 1]:
We studied 40 patients with premature canities and an equal number of age and sex-matched healthy controls. The number of males and females in the study group were equal (20 each) and ages ranged from 19–25 years. The earliest age at onset of canities was 9 years and the mean age of onset was 19.5 ± 2.9 years. The mean duration of greying was 34.5 ± 35.1 months (range 2–180 months). The mean grey hair count was 32.9 ± 15.8 and 13 (32.5%) patients had mild, 20 (50%) had moderate and 7 (17.5%) had severe canities. The parietal scalp (n = 30; 75%) was most commonly involved, followed by the temporal (n = 18; 45%), frontal (n = 11; 27.5%) and occipital (n = 2; 5%) areas. Greying of the beard hair was noted in four patients, but none had greying of axillary, chest or pubic hair. A family history of premature canities was seen in 11 patients (27.5%) and younger siblings were also afflicted in two cases. The affected parent was the father in eight cases.
| Parameter | Cases (n= 40) | Controls (n= 40) | P-value |
|---|---|---|---|
| Age (years) | 22.4 ± 2.31 | 22.4 ± 2.08 | – |
| Gender Males Females |
20 (50%) 20 (50%) |
20 (50%) 20 (50%) |
– – |
| Cardiovascular symptoms | 3 (7.5%) | 0 (0%) | 0.24 |
| Family history of premature canities | 11 (27.5%) | 0 (0%) | <0.001 |
| Family history of hypertension | 10 (25%) | 4 (10%) | 0.13 |
| Family history of diabetes mellitus | 11 (27.5%) | 3 (7.5%) | 0.03 |
| Weight (kg) | 64.18 ± 11.70 | 61.40 ± 10.95 | 0.37 |
| BMI (kg/m2) | 24.41 ± 3.66 | 23.30 ± 3.74 | 0.13 |
| Waist circumference (cm) | 83.80 ± 5.43 | 80.95 ± 6.51 | 0.05 |
| Normal BP | 11 (27.5%) | 20 (50.0%) | 0.03 |
| Elevated BP | 5 (12.5%) | 6 (15.0%) | 0.99 |
| Stage I hypertension | 23 (57.5%) | 14 (35.0%) | 0.04 |
| Stage II hypertension | 1 (2.5%) | 0 (0.0%) | – |
| Mean SBP | 119.4 ± 7.6 | 114.8 ± 6.2 | <0.01 |
| Mean DBP | 78.2 ± 5.9 | 72.9 ± 6.9 | <0.01 |
| Metabolic syndrome | 11 (27.5%) | 1 (2.5%) | <0.01 |
There were no significant differences between the study and control groups with regard to cardiovascular symptoms (shortness of breath, palpitations and chest pain), smoking, alcohol intake, family history of hypertension, mean weight, body mass index (BMI) and waist circumference except Stage I hypertension [as defined by the American Heart Association (AHA) criteria] where the difference was significant (P-value = 0.04).
Biochemical parameters [Table 2]:
The two groups were also similar with respect to mean serum cholesterol, low-density lipoprotein, very low-density lipoprotein and triglyceride levels, the mean high-density lipoprotein. The mean fasting blood sugar was significantly raised in cases as compared to controls (P-value < 0.01). The mean serum insulin levels were also significantly raised in patients as compared to controls (P-value < 0.001) with the maximum serum insulin levels in cases being 31.1 IU/mL compared to 19.3 IU/mL in controls. Fasting hyperinsulinemia was found in 8 patients (20%) but none of the controls (P-value < 0.01). The mean HOMA-IR was raised in patients with premature canities as compared to the controls (P-value < 0.001) with the HOMA-IR ranging from 0.3 to 7.7 in cases as compared to 1–5 in controls. Metabolic syndrome (as defined by the International Diabetes Federation criteria) was seen in 11 (27.5%) of the 40 patients with premature canities but in only a single control (P-value < 0.01) [Figure 1].
| Parameter | Cases (n= 40) | Controls (n= 40) | P-value |
|---|---|---|---|
| Total cholesterol (mg/dL) | 166.18 ± 33.59 | 154.53 ± 23.13 | 0.07 |
| LDL (mg/dL) | 99.00 ± 20.58 | 97.45 ± 27.39 | 0.39 |
| TG (mg/dL) | 113.58 ± 45.32 | 95.33 ± 25.15 | 0.12 |
| HDL (mg/dL) | 41.85 ± 10.57 | 45.01 ± 9.22 | 0.04 |
| VLDL (mg/dL) | 22.85 ± 8.88 | 19.08 ± 5.01 | 0.11 |
| Elevated cholesterol | 4 (10%) | 0 (0%) | 0.11 |
| Elevated triglycerides | 8 (20%) | 2 (5%) | 0.08 |
| Low HDL | 27 (67.5%) | 22 (55%) | 0.35 |
| Fasting blood sugar (mg/dL) | 96.43 ± 7.76 | 91.58 ± 6.53 | <0.01 |
| Serum insulin (IU/mL) | 16.37 ± 7.66 | 10.44 ± 3.46 | <0.001 |
| HOMA-IR | 3.97 ± 1.97 | 2.38 ± 0.89 | <0.001 |
| Impaired fasting glucose | 16 (40%) | 3 (7.5%) | <0.01 |
| Elevated serum insulin | 8 (20%) | 0 (0%) | <0.01 |
| hs-CRP (mg/L) | 1.70 ± 1.05 | 0.86 ± 0.71 | <0.001 |
| IL-6 (pg/mL) | 15.72 ± 48.40 | 3.98 ± 2.41 | 0.14 |
| TNF-α (pg/mL) | 19.42 ± 34.78 | 8.04 ± 4.32 | 0.43 |
| Elevated hs-CRP | 5 (12.5%) | 0 (0%) | 0.05 |
| Elevated IL-6 | 8 (20%) | 0 (0%) | <0.001 |
| Elevated TNF-α | 8 (20%) | 3 (7.5%) | 0.19 |
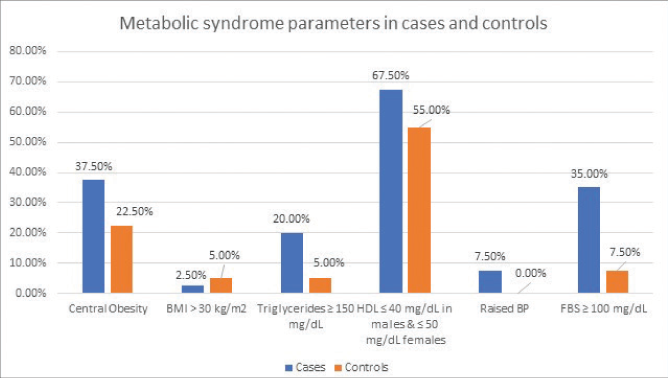
- Distribution of various components of International Diabetes Federation criteria of metabolic syndrome
The hs-CRP in cases ranged from 0.3 to 3.6 mg/L and in controls from 0.1 to 2.5 mg/L. The mean IL-6 and TNF-α levels were not significantly raised in the study group as compared to the controls. Elevated levels of IL-6 and hs-CRP were seen in 8 and 5 patients respectively but none of the controls, and elevated serum TNF-α levels were seen in 8 patients compared with 3 controls (P-value < 0.001).
Discussion
Premature canities in Asians is defined as the onset of greying of hair before the age of 25 years.1 Earlier studies have emphasized the deranged lipid profile and hyperinsulinemia and increased incidence of coronary artery disease in young patients with premature greying of hair10,11. The rising incidence of atherosclerosis among young patients in the Indian subcontinent is alarming, and we undertook the study to see if premature canities could be a marker of an elevated cardiovascular risk - if so preventive measures in such patients could lessen the disease burden secondary to coronary artery disease.
The male to female ratio of 1:1 in our study is in concordance with other studies from India.12-14 The prevalence of premature canities in family members in our study (27.5%) was similar to Mediratta et al.15 who observed a family history of premature canities in 29% of their patients. Paternal inheritance was more common than maternal inheritance in our study as was observed by Shin et al.16 who hypothesised that genetic factors including antioxidant factors are inherited from the father, thus the paternal history of canities is more common. The pattern of involvement of the scalp (parietal most commonly, occipital infrequently) seen in our patients was also noted by Sharma et al.17 and Russa et al.18
Sheikh et a1.19 have earlier noted that patients with premature greying had a higher mean BMI and this was seen in our patients too (P-value = 0.13). The mean waist circumference (an indirect marker of increased cardiovascular risk and a component of metabolic syndrome) of our patients indicates increased cardiovascular risk in individuals with premature canities.
Although 23 patients had stage I hypertension as compared to 14 controls the difference was not statistically significant. However, the mean systolic and diastolic blood pressure of cases was significantly higher than that of controls (P-value < 0.01). Paik et al.20 also commented on the elevated blood pressure in patients with premature greying of hair.
The significantly increased mean fasting blood sugar noted in our study is in agreement with earlier studies.10,20,21 Impaired fasting glucose was significantly more in patients with premature canities (P-value < 0.01). Aggarwal et al.11 have also reported a higher incidence of impaired fasting glucose and pre-diabetes in cases. Patients with premature canities had a higher mean serum insulin level and fasting hyperinsulinemia was significantly more in the patient group as compared to the control group.
In our study, a higher number of patients showed a deranged fasting lipid profile which was in concordance with other studies and signifies the elevated cardiovascular risk in these patients. The significant number of patients with metabolic syndrome as compared to controls (P-value < 0.01) is noteworthy, and has been earlier reported.20,22
Homeostasis model assessment of insulin resistance is a commonly employed surrogate marker of insulin resistance. It was first developed by Mathews and coworkers in 1985.23 Esteghamati et al.24 have concluded that with an increase in homeostasis model assessment of insulin resistance percentile, there is an increased risk of metabolic syndrome. The mean homeostasis model assessment of insulin resistance of cases was significantly elevated as compared to the controls (P-value < 0.001). Our results are in agreement with that of Chakrabarty et al.25 who noted a mean homeostasis model assessment of insulin resistance of 2.1 ± 1.9 and 1.7 ± 1.4 in patients with premature canities and controls, respectively.
Erdogan et al.21 found a significantly raised CRP in their cases as compared to controls, similar to our study. Since hs-CRP denotes ongoing inflammation and is an approved marker for increased cardiovascular risk, these results indicate that cases with premature canities are at an average to high risk of developing cardiovascular events in future.
Mean IL-6 level in patients was more than controls (P-value = 0.14) [Figure 2]. More patients with premature canities that is, 20% (n = 8) had elevated IL-6 levels from the baseline as compared to none (n = 0) of the controls (P-value < 0.001). Mean TNF-α levels in cases and controls was 19.4 ± 34.8 pg/mL and 8 ± 4.3 pg/mL, respectively (P-value = 0.43) [Figure 2]. Elevated TNF-α level from baseline, was seen in 20% (n = 8) patients and 7.5% (n = 3) of controls, the difference being statistically insignificant (P-value = 0.19). Our study is the first to estimate levels of pro-inflammatory cytokines (IL-6 and TNF-α) which are important upstream mediators of various inflammatory processes in the body including atherosclerosis.
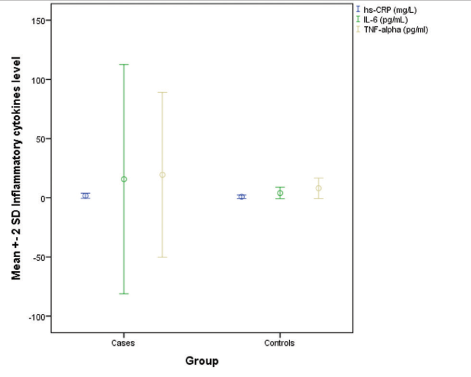
- Distribution of various inflammatory biochemical parameters among cases and controls
Therefore, IL-6 and TNF-α may serve as useful scientific markers for identifying individuals with premature canities who are at an increased risk of developing atherosclerosis and adverse cardiovascular events but further large-scale studies are needed to corroborate these findings.
Those patients found to have metabolic syndrome or subclinical insulin resistance on testing were advised lifestyle modifications in the form of dietary alterations like a Mediterranean diet or Dietary approaches to stop hypertension diet and exercise or regular physical activity for at least 30 minutes on most days of the week.26 Mediterranean diet is rich in whole-grain cereals, fruits, vegetables, and olive oil. Dietary approaches to stop hypertension diet advises against the use of high-fat dairy products and saturated fats.
Our study had few limitations. The sample size was small and we used a subjective scale for grading the severity of canities.
Conclusion
We studied cardiovascular risk in young patients with premature canities and found a significant association between the two. Screening of patients with premature canities at their first clinic visit with simple clinical parameters like blood pressure, body mass index and waist circumference and haematological parameters like hs-CRP, fasting lipid profile, blood sugar and serum insulin can help stratify individuals into low risk and high-risk groups enabling targeting preventive measures to the latter. IL-6 and TNF-α indicating an ongoing state of chronic inflammation in the body were elevated in patients of premature canities and may highlight patients at risk for atherosclerosis in future.
Declaration of patient consent
Patients’ consent is not required as the patients’ identity is not disclosed or compromised.
Conflict of interest
There are no conflicts of interest.
Financial support and sponsorship
Nil.
References
- Graying: Gerontobiology of the hair follicle pigmentary unit. Exp Gerontol. 2001;36:29-54.
- [CrossRef] [PubMed] [Google Scholar]
- Mechanisms of hair graying: Incomplete melanocyte stem cell maintenance in the niche. Science. 2005;307:720-4.
- [CrossRef] [PubMed] [Google Scholar]
- Towards a "free radical theory of graying": Melanocyte apoptosis in the aging human hair follicle is an indicator of oxidative stress induced tissue damage. FASEB J. 2006;20:1567-9.
- [CrossRef] [PubMed] [Google Scholar]
- Cutaneous photobiology. The melanocyte vs. the sun: Who will win the final round? Pigment Cell Res. 2003;16:434-47.
- [CrossRef] [PubMed] [Google Scholar]
- Protective effect of superoxide dismutase against hair graying in a mouse model. Photochem Photobiol. 2004;80:579-82.
- [CrossRef] [PubMed] [Google Scholar]
- Relationships between perceived workload, stress and oxidative DNA damage. Int Arch Occup Environ Health. 2001;74:153-7.
- [CrossRef] [PubMed] [Google Scholar]
- Accelerated telomere shortening in response to life stress. Proc Natl Acad Sci U S A. 2004;101:17312-5.
- [CrossRef] [PubMed] [Google Scholar]
- Redox-modulated pathways in inflammatory skin diseases. Free Radic Biol Med. 2001;30:337-53.
- [CrossRef] [PubMed] [Google Scholar]
- Antioxidant enzymes and lipid peroxidation in the scalp of patients with alopecia areata. J Dermatol Sci. 2002;29:85-90.
- [CrossRef] [PubMed] [Google Scholar]
- Premature graying of hair: An independent risk marker for coronary artery disease in smokers-A retrospective case control study. Ethiop J Health Sci. 2015;25:123-8.
- [CrossRef] [PubMed] [Google Scholar]
- A retrospective case-control study of modifiable risk factors and cutaneous markers in Indian patients with young coronary artery disease. JRSM Cardiovasc Dis. 2012;1:1-8.
- [CrossRef] [PubMed] [Google Scholar]
- Epidemiological and investigative study of premature graying of hair in higher secondary and pre-university school children. Int J Trichology. 2013;5:17-21.
- [CrossRef] [PubMed] [Google Scholar]
- Profile of Indian patients with premature canities. Indian J Dermatol Venereol Leprol. 2016;82:169-72.
- [CrossRef] [PubMed] [Google Scholar]
- Demographic characteristics and association of serum Vitamin B12, ferritin and thyroid function with premature canities in Indian patients from an urban skin clinic of North India: A retrospective analysis of 71 cases. Indian J Dermatol. 2017;62:304-8.
- [Google Scholar]
- An observational, epidemiological study on pattern of clinical presentation and associated laboratory findings in patients of premature hair graying. Int J Trichology. 2018;10:93-5.
- [CrossRef] [PubMed] [Google Scholar]
- Association of premature hair graying with family history, smoking, and obesity: A cross-sectional study. J Am Acad Dermatol. 2015;72:321-7.
- [CrossRef] [PubMed] [Google Scholar]
- Association of epidemiological and biochemical factors with premature graying of hair: A case-control study. Int J Trichology. 2018;10:211-17.
- [CrossRef] [PubMed] [Google Scholar]
- Association between premature hair greying and metabolic risk factors: A cross-sectional study. Acta Derm Venereol. 2018;98:748-52.
- [CrossRef] [PubMed] [Google Scholar]
- Relationship between trace elements and premature hair graying. Int J Trichology. 2018;10:278-83.
- [CrossRef] [PubMed] [Google Scholar]
- Premature hair graying: Profiling the scalp anatomical patterns of occurrence in Tanzania. Int J Anat Res. 2017;5:4635-39.
- [CrossRef] [Google Scholar]
- Premature hair whitening is an independent predictor of carotid intima-media thickness in young and middle-aged men. Intern Med. 2013;52:29-36.
- [CrossRef] [PubMed] [Google Scholar]
- Metabolic Syndrome in the spectrum of hair graying. Med J Basrah Univ. 2017;35:71-7.
- [CrossRef] [Google Scholar]
- Homeostasis model assessment: Insulin resistance and ß-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412-9.
- [CrossRef] [PubMed] [Google Scholar]
- Optimal cut-off of homeostasis model assessment of insulin resistance (HOMA-IR) for the diagnosis of metabolic syndrome: Third national surveillance of risk factors of non-communicable diseases in Iran (SuRFNCD-2007) Nutr Metab (Lond). 2010;7:1-8.
- [CrossRef] [PubMed] [Google Scholar]
- Factors associated with premature hair graying in a young Indian population. Int J Trichology. 2016;8:11-4.
- [CrossRef] [PubMed] [Google Scholar]
- Nutritional challenges in metabolic syndrome. J Clin Med. 2019;8:1301.
- [CrossRef] [PubMed] [Google Scholar]






