Translate this page into:
Change in spectrum of dermatophytes isolated from superficial mycoses cases: First report from Central India
2 Department of Dermatology and Venereology, Era's Lucknow Medical College and Hospital, Lucknow, Uttar Pradesh, India
Correspondence Address:
Sanjeev Sahai
Department of Microbiology, Teerthankar Mahaveer Medical College & Research Centre, Delhi Road, Bagarpur Moradabad- 244 001, Uttar Pradesh
India
| How to cite this article: Sahai S, Mishra D. Change in spectrum of dermatophytes isolated from superficial mycoses cases: First report from Central India. Indian J Dermatol Venereol Leprol 2011;77:335-336 |
Sir,
Superficial mycoses include fungal infections of skin, hair and nails caused predominantly by dermatophytes, yeasts and molds. Dermatophytes are anthropophilic, zoophilic and geophilic fungi, classified into three genera - Trichophyton spp. (T.), Microsporum spp. (M.) and Epidermophyton spp. (E.). Few species of dermatophytes (M. ferrugineum, T. soudanense etc.) are endemic in certain parts of the world and they are rarely encountered in other parts of the world. [1] Distribution of the dermatophytes varies with the geographical area and during the course of time. [2] We are observing a change in the spectrum of dermatophytic isolates from superficial mycoses cases in our region, and therefore the present study was carried out to know the changing epidemiology of dermatophytes, with special reference to M. ferrugineum a rare isolate in India.
Microsporum ferrugineum, an anthropophilic dermatophyte is endemic in Africa and Oriental Asia; sporadic cases have been reported from other countries, [2] only six cases have been reported so far from North-East India. [3]
In a retrospective study from August 2008 to September 2009, a total of 165 specimens were processed from clinically suspected cases of superficial mycoses attending the outpatient department of Dermatology and Venereology of our hospital. All specimens were examined by 10% KOH mount for screening of fungal elements and inoculated on duplicate Sabouraud′s Dextrose Agar (SDA) with 0.05 mg/ml chloramphenicol (with or without 0.5 mg/ml cycloheximide) at 25C in a BOD incubator for three weeks. Fungal isolates were identified according to standard procedures. [1] Laboratory-confirmed cases either by direct microscopy or culture were included in the data analysis and cases negative in both KOH and culture were excluded from the study. M. ferrugineum was identified on the basis of colony morphology on SDA medium, supplemented with chloramphenicol 0.05 mg/ml and cycloheximide 0.5 mg/ml [[Figure - 1]a], characteristic microscopic findings - ′bamboo hyphae′ with chlamydoconidia and lack of macro and microconidia in Lactophenol Cotton Blue (LCB) mount of slide culture [[Figure - 1]b], negative in-vitro hair perforation test, positive urease test and production of yellow pigment of Lowenstein Jensen medium. [1]
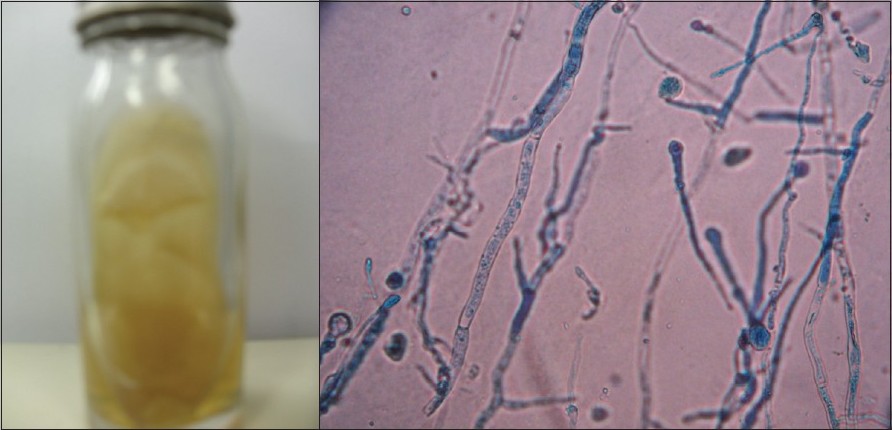 |
| Figure 1: (a)Folded, velvety, yellowish colony of Microsporum ferrugineum on Sabouraud's dextrose agar Figure 1b: Lactophenol Cotton Blue (LCB) mount of Microsporum ferrugineum showing characteristic microscopic findings - 'bamboo hyphae' with chlamydoconidia and lack of macro and microconidia (×400) |
Out of 165 cases enrolled, 148 (89.6%) cases were positive by direct microscopy and 137 (83%) cases were culture positive. Male : female ratio was 2 : 1, majority of patients belonged to 21-30 (32.4%) followed by 1-10 (21.6%), 11-20 (18.9%), 31-40 (13.5%) age groups, 114 (77%) cases belonged to low socioeconomic status and 90% cases were from sub-urban background. Most common clinical presentation was tinea corporis (45%) followed by T. capitis (34%), T. unguium (11%), T. manuum (5%), T. cruris (3%) and T. pedis (2%). All cases were immunocompetent and neither case had any history of traveling or staying abroad nor had any unusual clinical presentation. Treatment response could not be recorded due to poor patient compliance.
In our study, dermatomycoses was seen in 90% cases and superficial candidiasis in 10% cases. In 137 culture positive isolates most common dermatophyte isolated was T. mentagrophytes (25%) followed by T. tonsurans (20%), T. verrucosum (10.5%), M. ferrugineum (9.5%), T. schoenleinii (7%), M. audouinii (5%), T. rubrum (5%) and E. floccosum (3%), non-dermatophytic molds namely Aspergillus spp., Alternaria spp., Fusarium spp.. and Scopulariopsis spp. and Candida spp. were isolated from 5% and 10% cases, respectively. All Candida isolates were found to be of non-albicans type. M. ferrugineum was isolated from 13 cases - tinea corporis 4 (31%) was the most common presentation followed by T. manuum 3(23%), T. capitis, T. unguium and T. pedis 2 (15% each). Six (46%) and four (31%) cases were from suburban and rural background, respectively, and three (23%) from neighboring districts.
Singal et al., in a study of T. capitis cases from North India reported a change in the spectrum of dermatophytes with most common isolate as T. violaceum (38%) followed by M. audouinii, T. schoenleinii, T. tonsurans, M. gypseum, T. verrucosum and T. mentagrophytes.[4] We are first to report a change in the spectrum of the dermatophytes from Central India as well as one of the highest incidences of M. ferrugineum (9.5%) in the non-endemic part of the world; other studies have reported incidence rates between 0.01 and 7.3%. M. ferrugineum usually causes juvenile T. capitis, but recently it has also been isolated from cases of T. corporis, T. manuum etc. [5],[6] Our findings are also in accordance with these studies; however we are first to report two cases of T. unguium due to M. ferrugineum, which rarely infects nails.
In India, till date only one study by Grover and Roy has reported six (5.8%) cases of M. ferrugineum from North-East India and interestingly they did not isolate it from any T. capitis case. [3] We hypothesize that the possible route of entry of M. ferrugineum in India might be from its North-East border, which adjoins Myanmar - an endemic region for this pathogen. Out of 13 cases, six (46%) belonged to urban background, four (31%) of rural background and three (23%) from neighboring districts, which suggests a fair distribution and endemicity of this pathogen in our region.
We can conclude that India is a growing economy and during last couple of decades people have started interstate migration in search of better jobs, and foreign tourists are also visiting India more often than ever; therefore, change in spectrum of dermatophytes and uncommon fungal isolates can be encountered in clinical practice, and it would be prudent for medical mycologists to be well equipped to diagnose such cases.
| 1. |
Rippon JW. Dermatophytosis and dermatomycoses In: Medical mycology-The pathogenic fungi and actinomycetes. 3 rd ed. Philadelphia: W.B. Saunders company; 1988 p. 169-275.
[Google Scholar]
|
| 2. |
Seebacher C, Bouchara JP, Mignon B. Updates on the epidemiology of dermatophyte infections. Mycopathologia 2008;166:335-52.
[Google Scholar]
|
| 3. |
Grover S, Roy P. Clinico-mycological profile of superficial mycosis in a Hospital in North East India. Med J Armed Forces Ind 2003;59:114-6.
[Google Scholar]
|
| 4. |
Singal A, Rawat S, Bhattacharya SN, Mohanty S, Baruah MC. Clinico-mycological profile of tinea capitis in North India and response to griseofulvin. J Dermatol 2001;28:22-6.
[Google Scholar]
|
| 5. |
Neji S, Makni F, Cheikhrouhou F, Sellami A, Sellami H, Marreckchi S, et al Epidemiology of dermatophytoses in sfax, Tunisia. Mycoses 2009;52:534-8.
[Google Scholar]
|
| 6. |
Ngwogu AC, Otokunefor TV. Epidemiology of dermatophytes in a rural community in Eastern Nigeria and review of literature from Africa. Mycopathologia 2007;164:149-58.
[Google Scholar]
|
Fulltext Views
3,375
PDF downloads
2,399





