Translate this page into:
Chemokine receptors CCR5 and CCR2 genes in HIV positive, HIV exposed seronegative and in HIV unexposed individuals: A study from Mumbai
2 Department of Microbiology, Seth G.S. Medical College and K.E.M Hospital, J.M. Street, Parel, Mumbai, Maharashtra, India
Correspondence Address:
Jayanti Mania-Pramanik
Department of Infectious Diseases Biology, National Institute for Research in Reproductive Health, (Indian Council of Medical Research), Mumbai - 400 012, Maharashtra
India
| How to cite this article: Chaudhari DV, Kerkar SC, Chavan V, Mehta PR, Mania-Pramanik J. Chemokine receptors CCR5 and CCR2 genes in HIV positive, HIV exposed seronegative and in HIV unexposed individuals: A study from Mumbai. Indian J Dermatol Venereol Leprol 2015;81:548 |
Sir,
Discordant couples are a group of individuals who are considered as a good model to evaluate the association of different host factors with human immunodeficiency virus (HIV) transmission. The negative spouse of discordant couples are repeatedly exposed to their HIV infected spouse, however, they remain uninfected. Studies had established the association of homozygous deletion of β-chemokine receptor gene CCR5 (CCR5Δ32) and mutation in CCR2 V64I genes with HIV transmission. [1],[2] No study is reported on the association of these two chemokine receptors with HIV infection from western India and particularly from Mumbai. Hence, a prospective case control study was conducted during the years 2008-2011 with approval of the Institutional Ethics Committee and Hospital Ethics Committee to evaluate (i) these mutations among the western Indian population in Mumbai; (ii) the frequency of CCR5Δ32 and viral load with HIV transmission among sero-discordant couples and (iii) CCR2 V64I polymorphism frequency and its association with delay in disease progression among the HIV infected spouse.
Individuals or couples attending the Integrated Counseling and Testing Centre (ICTC) of Seth G.S. Medical College and KEM Hospital in Mumbai for HIV screening, either voluntarily or as referred cases, were enrolled with informed consent. Study groups were (i) serodiscordant couples (n = 35); their clinical history was considered to enroll them as discordant couples (ii) concordant couples (reference control, n = 35) and (iii) healthy individuals (control, n = 25).
Blood was collected for confirmation of HIV status, viral load assay and polymerase chain reaction (PCR) for polymorphism of CCR5 and CCR2 genes. HIV antibody testing was done as per the World Health Organization (WHO) guidelines. Seronegative spouses were screened at regular intervals using three rapid tests and confirmed by the qualitative HIV DNA polymerase chain reaction test. This test confirmed that all the HIV positive individuals, including the seroconverter, were positive and the known negative controls as well as the seronegative spouses were negative at all follow-up visits.
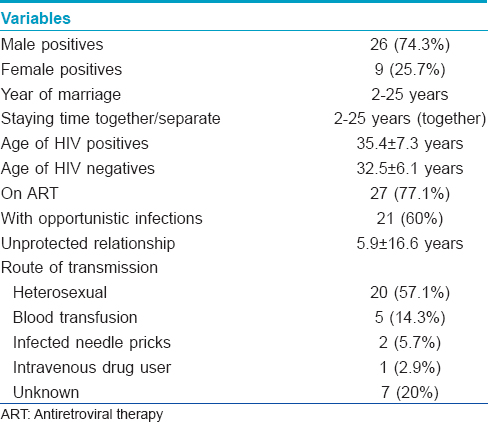
Clinical history of the discordant couples who completed 2 years follow-up revealed that they were staying together and had unprotected coitus before and after one of the couple was detected to be HIV positive. Follow-up co-operation was obtained only in 35 (27.1%) of 129 of the identified discordant couples [Figure - 1]. The details of these couples is presented in [Table - 1]. The screening for HIV detection in the positive spouse was done from 1998 to 2009 (duration: 2-11 years). Screening of the other spouse was done between the years 2004 and 2009. The gap between the HIV detection in one spouse and screening of the other spouse varied from 0 to 9 years. In 9 couples HIV screening of the other spouse was done immediately (at 0 months). Twenty seven had unprotected coitus even after counseling. They had unprotected sex (at least 2-3 times or more) within 6-7 months prior to enrollment. The remaining serodiscordant couples (n = 8) did not consistently use protective methods. The frequency of coitus/vaginal intercourse varied from 1 to 3 episodes per month. In the discordant group, 27 were started on antiretroviral treatment. Thirteen of them had a history of tuberculosis infection.
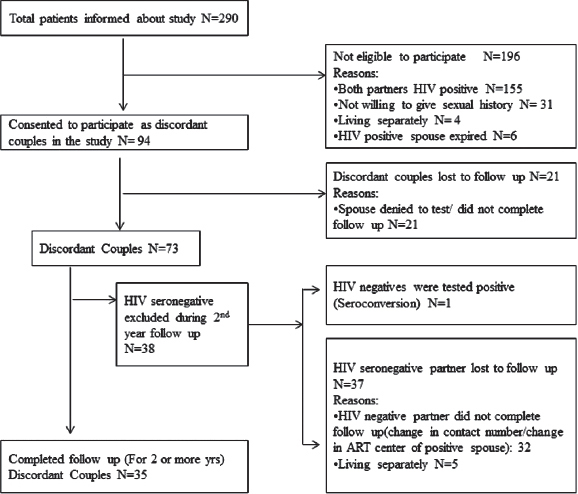 |
| Figure 1: Enrolment details of the present study participants |
The CCR5 32 deletion was genotyped by detecting size differences in the amplicons on 3% agarose gel; wild type (WT) CCR5 gene with 189 bp product, homozygote CCR5 mutant gene with 157 bp, and the heterozygote CCR5 gene with both 189 and 157 bp products. [3] No homozygous or heterozygous deletion was observed in CCR5 gene; all were with wild type gene [Figure - 2]a. Our observation on complete absence of CCR5Δ32 deletion is consistent with a report from a north Indian population. [4] Although a previous study has shown a 2% heterozygous CCR5Δ32 deletion in 300 studied women, these 6 women belonged to the Muslim community, [3] as was reported among the Muslims of northern India. In the present study none of the subjects were Muslim. The exposed spouse having wild type CCR5 gene remained negative in spite of repeated exposures. Above all, the report on the occurrence of HIV-1 infection in an individual homozygous for CCR5Δ32 gene indicates a controversial role of this CCR5Δ32 deletion in HIV transmission. [5]
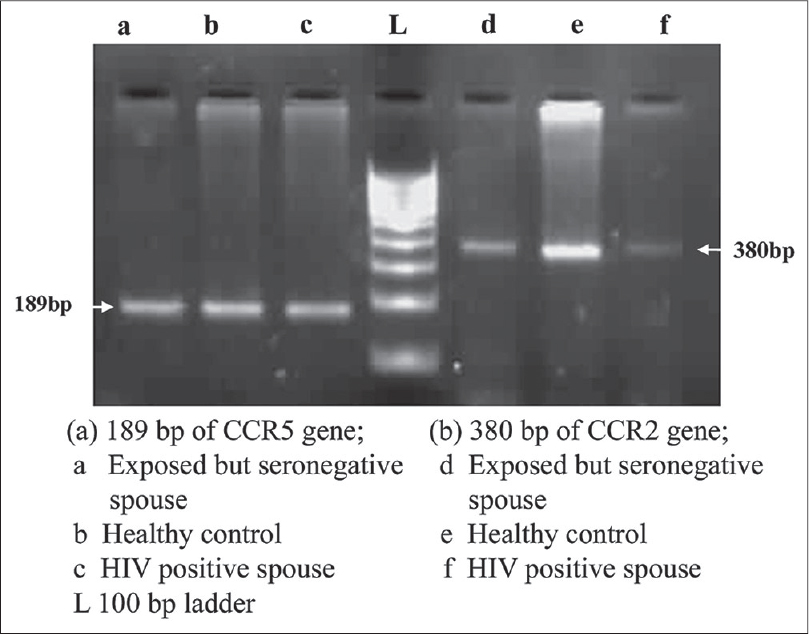 |
| Figure 2: Amplification product of CCR5 (189 bp), and CCR2 gene (380 bp) |
The amplified products of CCR2 gene size was of 380 bp, no valine to isoleucine substitution was observed in any of these groups and all subjects showed wild type gene [Figure - 2]b. In contrast, to the earlier reports from India, [6] this is the first report from western India, indicating complete absence of CCR2 V64I polymorphism in the studied population.
All the enrolled HIV-positive individuals were advised to follow up for CD4 count estimation. They were grouped into two, on the basis of their CD4 count (not given here) as progressors (PR) or slow progressors (SP) toward acquired immune deficiency syndrome (AIDS) [Table - 2]. If the CD4 count declined (<250 cells/mm 3 ) within a year of HIV detection, they were grouped as progressors and if the count was more than >250 cells/mm 3 for more than 3 years after HIV detection or there was insignificant difference in CD4 counts between two dates, they were grouped as slow progressors. Increase or decrease in CD4 count was taken into consideration while associating response with treatment [Table - 2]. Follow up of the HIV positive individuals revealed that out of 105 individauls 40 (38.1%) were progressing towards AIDS (HIV detection period: 1-3 years) and 67 (63.8%) were on treatment of whom 27 (40.3%) did not respond to the treatment. Among the progressors (n = 40), 16 of 27 (59.3%) were responding to the treatment. Among the slow progressors (n = 65, HIV detection period: 2-5 years), 40 (61.5%) were on treatment and 24 were responding to treatment. Thirty eight HIV positive individuals were not under any medication though some were infected between 1 to 7 years ago. CCR2 V64I mutation substantially reduces the risk of HIV disease progression, however, only 40 (38.1%) of the 105 infected individuals showed a rapid decline of CD4 count suggesting progression towards AIDS. Low adherence to the treatment regimen might be associated with no response to medication. Among the 65 grouped as slow progressors, CD4 count had declined slowly over the years in 40 individuals to <250 cells/mm 3 while the other 25 individuals had normal CD4 count, suggesting possibly no influence of wild type CCR2 gene.
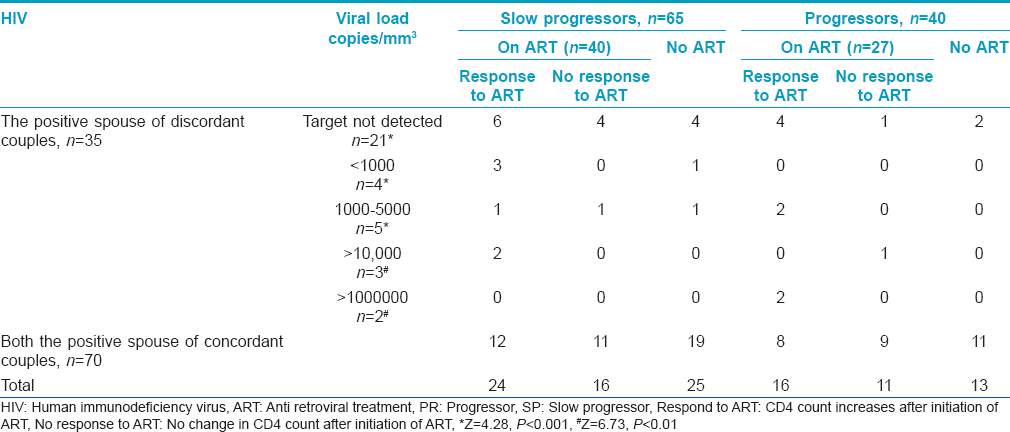
Viral load of the HIV positive spouse before antiretroviral therapy (pre-ART), their disease status as progressors or slow progressors and response to treatment is presented in [Table - 2]. The proportion of spouses with undetermined viral load was significantly high in comparison with others. Even when taken together, the proportion of spouses (21 of 35) with undetermined viral load was significantly high (Z = 2.85, P < 0.01) when compared with those with known viral load (14 of 35). One of the reasons for this trend seems to be due to awareness about the protection of the uninfected partner. Owing to the cost factor and also, since both partners were infected, concordant couples were not evaluated for viral load.
It is well established that in a population, host-related factors (susceptibility and infectiousness), environmental factors (social, cultural, and political milieu), and agent factors (HIV type 1) determine HIV infectivity. CD4 counts and viral load are the confounding factors responsible for HIV transmission as well as its progression. The levels of these two factors modulate the antiretroviral treatment. CD4+ T cell count is an important criterion for categorizing HIV-related clinical conditions. In the absence of specific deletion in CCR5 gene or mutation in CCR2 gene, the positive spouse might have a low level of HIV viral load even before the initiation of treatment (in our report 27 on ART after enrollment). The HIV negative spouse might have been protected from acquiring infection by the low viral load of the infected partners or by the presence of some other confounding factors not studied here. Our results indicate the difficulty in enrolling discordant couples as only 35 (27.1%) individuals who completed 2 years of follow up could be enrolled. We did not extend the sample size as 165 study subjects showed absence of deletion in CCR5 gene or mutation in CCR2 gene. The analysis was limited specifically to these two polymorphisms as they were reported to be associated with HIV infection in worldwide studies. [1],[2]
In conclusion, our findings indicate absence of deletion in CCR5 or mutation in CCR2 genes in the studied HIV positive and exposed negative population as well as in unexposed healthy controls belonging to western Indian population. In discordant couples, besides some unknown factors, undetermined (target not found) viral load of positive spouse might be the possible factor that prevents their repeatedly exposed spouse from HIV infection.
| 1. |
Liu R, Paxton W, Choe S, Ceradini D, Martin SR, Horuk R, et al. Homozygous defect in HIV-1 conception accounts genes resistance of some Multiply-exposed individuals to HIV-1 infection. Cell 1996;86:367-77.
[Google Scholar]
|
| 2. |
Frade JM, Llorente M, Mellado M, Alcamí J, Gutiérrez-Ramos JC, Zaballos A, et al. The Amino-terminal Domain of the CCR2 Chemokine Receptor Acts as Coreceptor for HIV-1 Infection. J Clin Invest 1997;100:497-502.
[Google Scholar]
|
| 3. |
Mania-Pramanik J, Kerkar SC, Vallabhadas A, Mehta PB, Salvi V. Does CCR5 gene-Δ32 deletion protect C. trachomatis infected Indian women from tubal pathology? Microbiol Res 2011;3:19-21.
[Google Scholar]
|
| 4. |
Kaur G, Singh P, Rapthap CC, Kumar N, Vajpayee M, Sharma SK, et al. Polymorphism in the CCR5 Gene Promoter and HIV-1 Infection in North Indians. Hum Immunol 2007;68:2454-61.
[Google Scholar]
|
| 5. |
Biti R, French R, Young J, Bennetts B, Stewart G, Liang T. HIV-1 infection in an individual homozygous for the CCR5 deletion allele. Nat Med 1997;3:252-3.
[Google Scholar]
|
| 6. |
Ramana GV, Vasanthi A, Khaja M, Sui B, Govindaiah V, Jini L, et al. Distribution of HIV-1 Resistance conferring polymorphic alleles SDF-1-3 A, CCR2-64 I and CCR5- Δ32 in diverse populations of Andhra Pradesh, South India. J Genet 2001;80:137-40.
[Google Scholar]
|
Fulltext Views
2,267
PDF downloads
2,012





