Translate this page into:
Cilostazol: A novel agent in recalcitrant livedoid vasculopathy
2 Department of Pathology, Lady Hardinge Medical College and Associated Hospitals, New Delhi, India
Correspondence Address:
Meenu Malik
C-51, Ayudh Vihar, Sector 13, Plot No. 3, Dwarka, Phase 1, New Delhi - 110 078
India
| How to cite this article: Mendiratta V, Malik M, Yadav P, Nangia A. Cilostazol: A novel agent in recalcitrant livedoid vasculopathy. Indian J Dermatol Venereol Leprol 2016;82:222-224 |
Sir,
A 25-year-old woman presented with recurrent erythematous, painful lesions over her lower extremities for 1½ years, some of which turned into slow-healing ulcers. There was no history of preceding drug intake or constitutional symptoms. Examination revealed multiple small (0.5-1.5 cm), extremely tender, linear to angulated superficial ulcers with necrotic margins and pale bases over the lower third of both legs and around the ankles [Figure - 1]. Ulcers were interspersed with erythematous to violaceous macules, stellate scars, focal induration and post-inflammatory hyperpigmentation, with associated mild pedal edema. Peripheral pulses were palpable.
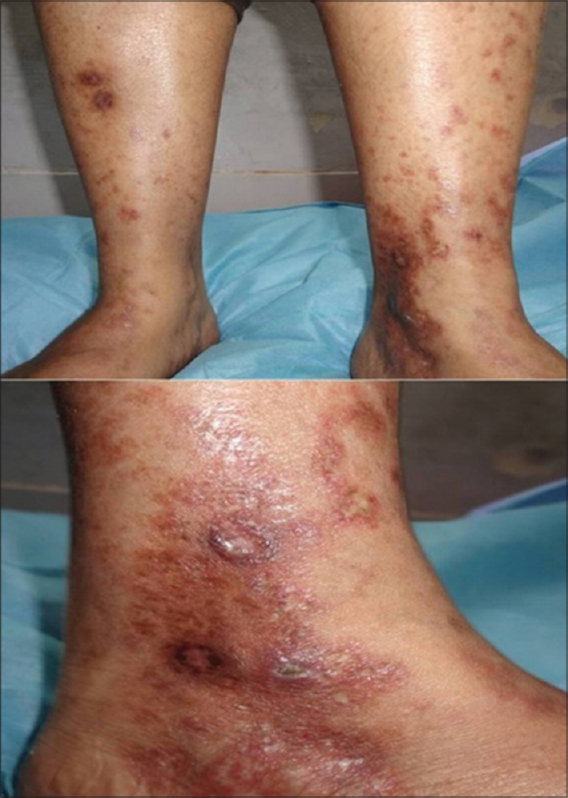 |
| Figure 1: Active livedoid vasculopathy (pre-treatment) |
Her hematological and biochemical parameters were normal. Autoantibody (antinuclear antibody, anti thyroid peroxidase antibody) tests were negative, and the thyroid profile, protein C and protein S levels were normal. No mutations in the factor V (Leiden) or prothrombin genes were detected. However, a heterozygous methylenetetrahydrofolate reductase (MTHFR) gene mutation with hyperhomocysteinemia and an increase in thrombin was noted. Venous and arterial Doppler studies of the lower limbs were normal. Histopathological findings confirmed the diagnosis of livedoid vasculopathy [Figure - 2] and [Figure - 3].
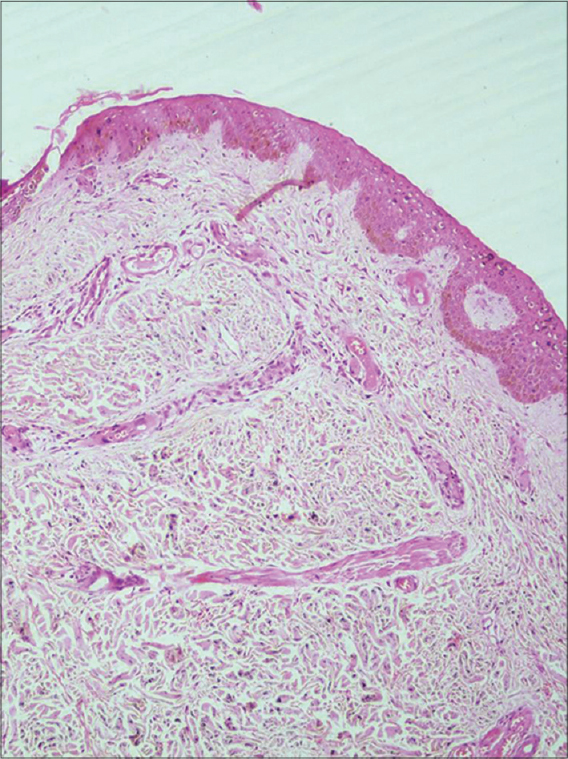 |
| Figure 2: Focal minimal perivascular mononuclear infiltrate in the superficial and mid-dermis with numerous foci of red cell extravasation (H and E, ×100) |
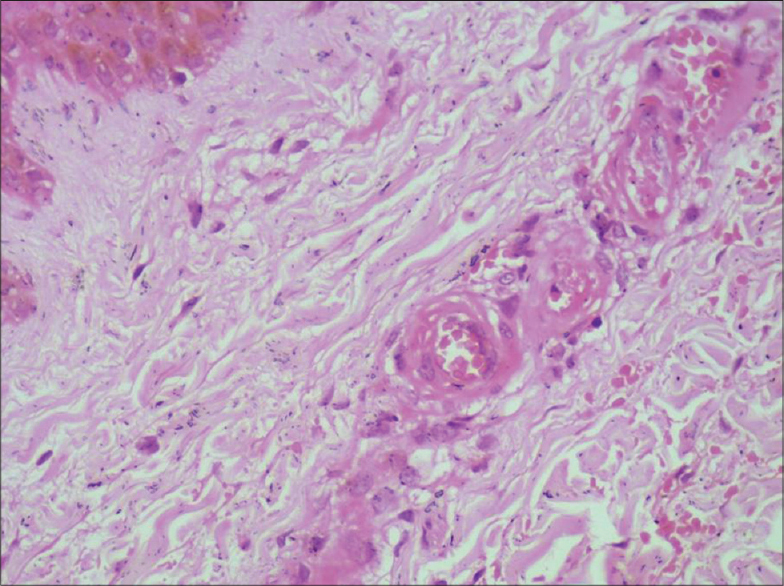 |
| Figure 3: Endothelial cell swelling and fibrinous deposits in the vessel wall. A few vessels also show luminal occlusion by thrombus (H and E, ×400) |
She had had a chronic, non-remitting, prolonged course unresponsive to multiple therapeutic agents. She was already on aspirin, 75 mg once daily and pentoxiphylline, 400 mg twice daily without control of disease activity. She had also previously received dapsone, oral corticosteroids, colchicine and subcutaneous low molecular weight heparin for several weeks without any significant improvement. Hence, oral cilostazol was added at a dose of 50 mg once daily. Within 2 weeks, she reported a significant reduction in pain and burning along with partial healing of the ulcers [Figure - 4]. No new lesions developed in the next 2 months. However, after 2 months, she experienced another flare which was managed by increasing the dose of cilostazol to twice daily. She again responded well and was subsequently maintained on the initial regime of cilostazol 50 mg once daily with aspirin and pentoxifylline for 3 months. The drugs were then gradually tapered off and there was no relapse for the next one year.
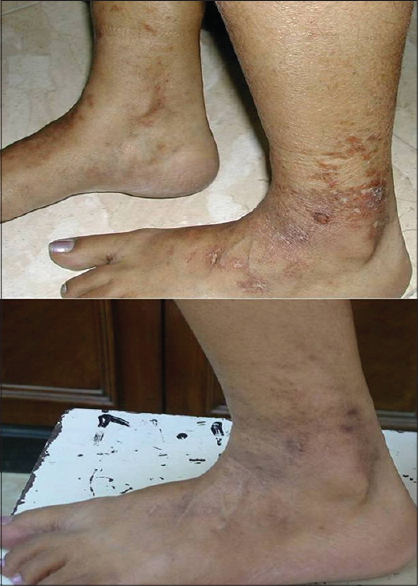 |
| Figure 4: Healed lesions with post-inflammatory hyperpigmentation (post-treatment) |
Livedoid vasculopathy is a chronic, recurrent occlusive vasculopathy of small dermal vessels characterized by recurrent painful, slowly healing ulcerations over the lower extremities resulting in porcelain-white, stellate, atrophic scars surrounded by hyperpigmentation and telangiectasias, referred as "atrophie blanche of Milian." It predominantly affects young and middle-aged females. Focal painful purpuric macules and/or papules on the legs may be seen in early cases.
Formation of microthrombi in the superficial dermal vessels secondary to a defect in endothelial cell plasminogen activator, platelet dysfunction or enhanced fibrin formation are considered possible pathogenic mechanisms. [1] Livedoid vasculopathy has been observed in patients with altered coagulation such as those with factor V Leiden mutation, protein C deficiency, antiphospholipid syndrome, increased plasma homocysteine levels, abnormalities in fibrinolysis, increase in platelet activation and sickle cell disease. [2] Our patient had an MTHFR gene mutation with hyperhomocysteinemia and increased thrombin levels as a predisposing condition. Focal deposition of fibrin in the lumen with thrombosis, segmental hyalinization and thickening of blood vessels in the superficial and mid-dermis, proliferation of endothelial cells and an absence of vasculitis are pathognomonic histopathological features.
There is no single efficacious treatment for livedoid vasculopathy. Etilestrenol, dapsone, corticosteroids, acetylsalicylic acid, clopidogrel, cilostazol, sildenafil, tadalafil, warfarin, low molecular weight heparin (LMWH), nifedipine, nicotinic acid, pentoxifylline, S2 serotoninergic blockers, cyclosporine, hyperbaric oxygen, danazol, iloprost, intravenous immunoglobulin and psoralen plus ultraviolet-A have all been tried with some benefit. Cilostazol is a potent inhibitor of phosphodiesterase (PDE) 3A, the isoform of PDE 3 present in platelets, vascular smooth muscle and heart. [3] It exhibits affinity for both cAMP and cGMP, but hydrolyses cAMP much more rapidly than cGMP. In addition, it inhibits adenosine uptake eventually resulting in changes in cAMP levels, dependent on the type of adenosine receptors (A1 or A2). Cilostazol is approved for the treatment of intermittent claudication due to its vasodilatory and antiplatelet actions. It inhibits platelet aggregation and has considerable antithrombotic effects in vivo, thus countering the main mechanism underlying livedoid vasculopathy. Further, it relaxes vascular smooth muscle and inhibits mitogenesis and migration of vascular smooth muscle cells. It is preferred over other antiplatelet agents as the disruption of primary and secondary platelet aggregation is short-lived. [4] Neither prolongation of bleeding time nor any coagulation abnormality was seen in our patient unlike as described with aspirin alone. [5] Our experience with this patient suggests that cilostazol may provide prolonged control of disease activity in refractory cases of livedoid vasculopathy.
| 1. |
Gonzalez-Santiago TM, Davis MD. Update of management of connective tissue diseases: Livedoid vasculopathy. Dermatol Ther 2012;25:183-94.
[Google Scholar]
|
| 2. |
Hairston BR, Davis MD, Pittelkow MR, Ahmed I. Livedoid vasculopathy: Further evidence for procoagulant pathogenesis. Arch Dermatol 2006;142:1413-8.
[Google Scholar]
|
| 3. |
Gresele P, Momi S, Falcinelli E. Anti-platelet therapy: Phosphodiesterase inhibitors. Br J Clin Pharmacol 2011;72:634-46.
[Google Scholar]
|
| 4. |
Iwamoto T, Kin K, Miyazaki K, Shin K, Takasaki M. Recovery of platelet function after withdrawal of cilostazol administered orally for a long period. J Atheroscler Thromb 2003;10:348-54.
[Google Scholar]
|
| 5. |
Comerota AJ. Effect on platelet function of cilostazol, clopidogrel, and aspirin, each alone or in combination. Atheroscler Suppl 2005;6:13-9.
[Google Scholar]
|
Fulltext Views
4,813
PDF downloads
2,435





