Translate this page into:
Clinical and epidemiological trends in childhood leprosy: A 20-year retrospective analysis from a tertiary care hospital in Jammu, North India
-
Received: ,
Accepted: ,
How to cite this article: Sakral A, Dogra N, Dogra D, Sharma K. Clinical and epidemiological trends in childhood leprosy: A 20-year retrospective analysis from a tertiary care hospital in Jammu, North India. Indian J Dermatol Venereol Leprol 2022;88:755-60.
Abstract
Background
Slightly more than half the total number of childhood leprosy cases worldwide are from India.
Aim
To analyze the clinical and epidemiological trends of childhood leprosy over 20 years in a tertiary care hospital.
Methods
We retrieved the medical records of all children less than 15 years of age registered in the leprosy clinic between April 1998 and March 2018. We tabulated and analyzed data pertaining to demographic details along with clinical findings such as cutaneous lesions, nerves involved, sensory loss, deformities, reactions, smear status, histopathology and treatment.
Results
Out of total 1548 leprosy cases registered during the study period, 55 (3.55%) cases of childhood leprosy were diagnosed. Thirty five (63.6%) children were in the age group of 11–15 years and 83.7% were migrants from other states. Thirteen (23.6%) children reported contact with a diagnosed case of leprosy, mainly in close contacts. Fifty three (96.4%) children presented with cutaneous lesions while 2 (3.6%) had pure neural involvement. Borderline tuberculoid leprosy was the most common clinical presentation in 27 (49.1%) followed by borderline lepromatous leprosy in 11 (18%). Thickened peripheral nerve trunks were detected in 42 (76.4%), most commonly the ulnar nerve. Reactional episodes occurred in 12 (21.8%) cases (Type 1 reaction, 10 (18.2%); Type 2 reaction, 2 (3.6%)). Grade 2 disability was detected in 4 (7.3%). Multidrug therapy was started in all patients, multibacillary (MB) regimen in 42 (76.3%) patients and paucibacillary (PB) regimen in 13 (23.7%). Twenty five (45.4%) children defaulted from the treatment. On comparing the data of 2008-18 with that of the previous decade (1998–2007), there was a higher proportion of migrant cases as compared to local cases (3:1–11:1) and MB cases as compared to PB cases (2:1–6:1). The proportion of treatment defaulters declined from 60% to 36%.
Limitations
Relapse rate could not be calculated due to inadequate follow-up period. As it is a hospital-based retrospective study with no active surveys, these findings may not reflect trends in the community.
Conclusion
Childhood leprosy continues to be a significant problem. There is a clear need to strengthen early detection, treatment and regular follow-up of these cases in both high and low endemic settings.
Keywords
Children
epidemiology
global
leprosy
national
update
Plain Language Summary
Leprosy in children indicates an active transmission of the disease in the community. One of the global targets set by the World Health Organisation is to reduce the burden of childhood leprosy and associated disability in the pediatric population. Although leprosy has achieved the status of elimination at both global and national levels, leprosy in children is yet to be eliminated. As this parameter is showing a very steady decline towards its set target, formulation of more effective measures along with proper monitoring is required to curb the chain of transmission in this age group.
Introduction
One of the three principal targets of the global leprosy strategy 2016–2020 proposed by the World Health Organization (WHO), is zero incidence of new cases of childhood leprosy with Grade 2 disability.1 These cases are considered an important epidemiological indicator as they reflect prolonged exposure of children to untreated “open cases” in the community from a very young age, with a long lag time from incubation till the appearance of symptoms. The incidence of childhood leprosy with Grade 2 disability is also used to monitor the operational efficiency of a national programme as its occurrence indicates the delayed diagnosis of open cases leading to an increase in associated complications.
At the global level, India contributes ~58% of the total child leprosy burden followed by Indonesia and Brazil. The global burden shows a gradual decline towards the set target of child leprosy.2 This may be due to hyperendemicity of leprosy in certain states despite achieving elimination at the national level, thereby, exposing this vulnerable population with immature immunity more toward the disease.
Jammu and Kashmir has a prevalence of leprosy well below the elimination level of <1 / 10,000 population since the declaration of leprosy elimination at the national level in 2005. However, several reports from India and abroad indicate that the prevalence of childhood leprosy is unchanged in many communities. This led us to analyze the data on childhood leprosy in our hospital records.
Methods
Participants and setting
This study was conducted in the postgraduate Department of dermatology, venereology and leprology, Government Medical College and Hospital, Jammu (Jammu & Kashmir, India), after receiving clearance from the Institutional Ethics Committee. Data was retrieved from the preformatted leprosy cards of leprosy cases registered from April 1998–March 2018 in the urban leprosy clinic attached to the department. Children <15 years of age who were diagnosed with leprosy as per WHO case definition were included.3
Data details
Data included the following variables: age, sex, educational status, permanent residential address, contact exposure, duration of symptoms, number of skin lesions, their distribution, morphology, associated symptoms (sensory loss and paresthesias), nerve involvement (number and distribution) associated neuritis, nerve abscess, disabilities and reactional episodes. The information regarding slit skin smear status at the time of presentation, after six months and at the end of the treatment and histopathological details were also documented. Considering the clinical details, cases were classified according to the Ridley–Jopling classification as tuberculoid (TT), borderline tuberculoid (BT), mid-borderline (BB), borderline lepromatous (BL) and pure lepromatous (LL) with additional classifications of pure neural and indeterminate types.4,5 The WHO operational classification and the one modified under NLEP, India, have been used to classify the patients into multibacillary (MB) and paucibacillary (PB) cases.6,7 Treatment details entailing the type of treatment regimen (PB-MDT or MB-MDT), whether released from treatment (RFT) or not, defaulter and relapse rate were also documented.
Data analysis
Data was managed and analyzed in Microsoft Excel and SPSS software was used for statistical analysis.
Results
A total of 1548 new cases of leprosy were diagnosed over the study period of 20 years, of which 55 (3.55%) cases were children <15 years of age.
The annual distribution of childhood cases is shown in Figure 1 and the age and sex distribution and bacilliferous status in Table 1. The mean age of study population was 11.82 years with a standard deviation of ±2.35 with 35 (63.6%) children aged between 11-15 years. There were 41 (74.5%) boys and 14 (25.6%) girls. There were 42 (76.4%) children with MB disease and 13 (23.6%) with PB disease.
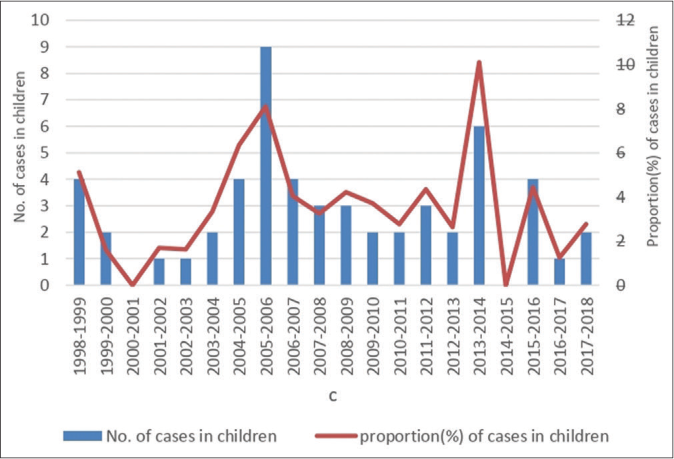
- Number of childhood cases and proportion of children <15 years with leprosy among all newly detected cases in the present study between 1998 and 2017
| Age (in years) |
Total no. of cases | Male | Female | ||||
|---|---|---|---|---|---|---|---|
| *PB | **MB | Total | PB | MB | Total | ||
| 0–5 | 1 | - | 1 | 1 | - | - | - |
| 6–10 | 19 | 4 | 9 | 13 | 3 | 3 | 6 |
| 11–15 | 35 | 4 | 23 | 27 | 2 | 6 | 8 |
| Total | 55 | 8 | 33 | 41 | 5 | 9 | 14 |
According to National Sample Survey, non-resident or a migrant is defined as a person residing in a place other than his or her place of birth or one who has changed his/her usual place of residence to another place. Depending on their duration of stay, migrants can be permanent, semi-permanent or long-term circular, seasonal or short-term circular.8 Nine (16.3%) of the children were permanent residents of Jammu while 46 (83.7%) were migrants. Most of the migrants were from the states of Chhattisgarh (14,25%) and Bihar (13,23%).
Household contacts are those who share the same dwelling area (i.e., kitchen, recreational area and living area). Extra household contacts include next door neighbours, friends, colleagues or even blood relatives who are not sharing the same home. Thirteen (23.6%) children reported contact with a diagnosed case of leprosy. Ten (76%) children reported exposure to an affected household contact, that is, parents and siblings while the other 3 (23%) revealed exposure with an extra-household contact, that is, a social contact.
Clinical presentation
The average duration of symptoms before presentation was 17.1 months. Symptoms were of <1 year duration in 31 (56.3%) children, between one and five years in 18 (32.7%) and more than five years in five (9.1%) children. Fifty three (96.4%) children presented with cutaneous symptoms: either hypopigmented, anesthetic patches (in the majority) or papulonodular lesions [Figure 2]. Only two (3.6%) showed pure neural involvement without any cutaneous lesion. Twenty two (40%) children had >5 skin lesions and the most common site of involvement was the limbs followed by face and trunk. Borderline tuberculoid (BT) leprosy was the most common clinical type in 27 (49%) children followed by borderline lepromatous (BL) leprosy in 11 (18%). There were five children with lepromatous leprosy (LL); the fathers of two of these children had leprosy and were on treatment in our institute [Table 2]. Clinically thickened peripheral nerve trunks were detected in 42 (76.4%) children, of whom 32 (58.3%) had involvement of more than one nerve. Ulnar nerve was the most common thickened nerve.

- Erythematous plaque with central clearing and sloping outer margins in mid-borderline leprosy (BB) with type 1 reaction.

- Ulnar claw hand deformity
| Clinical parameters | Number of cases, n (%) |
|---|---|
| Number of cutaneous lesions | |
| >5 lesions | 22 (40) |
| 2–5 lesions | 15 (27.2) |
| Single lesion | 16 (29) |
| *Clinical presentation | |
| BT | 27 (49) |
| BL | 10 (18.2) |
| BB | 8 (14) |
| LL | 5 (9) |
| Pure neural | 2 (3.6) |
| Indeterminate | 3 (5.4) |
| Peripheral nerves involvement | 42 (76.4) |
| Single nerve | 10 (18.1) |
| >1 nerve | 32 (58.3) |
| Classification | |
| MB | 42 (76.3) |
| PB | 13 (23.7) |
| Smear positivity | 14 (25.4) |
| Reactions | 12 (21.8) |
| Type 1 | 10 (18.2) |
| Type 2 | 2 (3.6) |
| Disability | |
| Grade 1 disability | 11 (20) |
| Grade 2 disability | 4 (7.3) |
| Treatment completion rate | 30 (54.4) |
Grade 2 disability manifesting as ulcers over hands and feet and ulnar claw hand was seen in 3 (5.4%) and 1 (1.8%) children respectively, with an overall Grade 2 disability rate of 7.3%. Reactional episodes developed in 12 (21.8%) children, of which 10 (18.2%) had type one reaction and two (3.6%) had type two reaction. Associated neuritis was detected in 3 (5.4%) patients. Two cases had severe neuritis with recent onset motor weakness, for which they were treated with oral steroids according to the body weight and the medication was tapered and finally stopped after periodic assessment of motor activity. Slit skin smears showed acid fast bacilli in 14 (25.4%) children. Out of 55 cases, skin biopsy record could be retrieved in 46 cases. Among these, clinico-histopathological correlation was established in 30 cases with borderline tuberculoid leprosy (BT) reported in 22 (47.8%) biopsies being the most common histopathological diagnosis. Overall, Fite-Faraco stain for acid fast bacilli was positive in nine (16.4%) biopsies.
Treatment was started in all newly diagnosed cases according to WHO guidelines.3 MB multidrug therapy was started in 46 (76.3%) and was continued for one year. However, in two cases, treatment was extended for an additional year depending on their slit skin smear status and clinically slow regression of cutaneous lesions at the end of one year. Thirty (54.5%) patients completed the adequate treatment and were released from treatment while 25 (45.5%) were found to be either first visit drop outs or defaulters, that is, they did not complete six months of treatment in nine months for PB cases and 12 months of treatment in 18 months for MB cases. Most of the dropouts and defaulters belonged to the PB category. Clinical parameters of the study population are summarized in Table 2.
Discussion
The global prevalence of leprosy at the end of 2017 including the data from India (till March 2018) was 0.25 (192,713 leprosy cases “on treatment”), annual new case detection rate (ANCDR) was 2.77 per 100,000 population (211,182 new cases) and the child case rate was 0.88 / 100,000 children ≤14 years of age (17,117 new child cases).2 During the same time period in India, according to the NLEP annual report (March 2018), prevalence rate per 10,000 population was 0.67 with the total case load of 90,709 and ANCDR was 9.27 per 100,000 population (126,614 new cases). Out of these, 10,287 cases were children <15 years of age amounting to the child proportion rate of 8.1%.9 From these figures, we can make out that India alone is contributing approximately 60% of total cases and 57.5% of total new child leprosy cases detected worldwide.
In the present study, an average child case rate of 3.55% over 20 years is quite comparable to the figures found in the study done by Dogra et al.10 (4.8%) in Chandigarh during 2001– 2011 and much less than the rate of 11.2% reported by Babu et al.11 in a study conducted in Mangalore between 2005 and 2015. During most of the reporting years, percentage of child leprosy cases remained >10% of newly detected cases in the Mangalore study, whereas in the Chandigarh study, the percentage hardly reached 10%. This could be due to the difference in geographical distribution of cases, determining the case load in a particular area and variation in the age cutoff criteria for the child category. Similar variation in the distribution of leprosy cases from area to area has also been demonstrated by Luna et al.12 and Santos et al.13
In accordance with studies undertaken in other centres,10,14,15 boys outnumbered girls in a ratio of 2.9:1.
In the current study, majority cases (63.6%) belonged to the age group of 11–15 years, corroborating with the observations made in earlier studies (59.3–71.1%).10,11,16 The percentage of children belonging to the age group of 6–11 years is quite considerable also (>30%). Moet et al. in their study observed that the risk of developing the disease between contacts increases from 5 to 15 years of age, peaking between 15 and 19 years of age.17 Scheelbeek et al. also reported a greater chance of disease in children and adolescents aged 8–14 years. The possible factors could be allied to the individual’s immunological response, genetic factors and the long incubation period of the disease.18
About 83.7% of children were non-residents of the state who were either accompanying their parents or their relatives, engaged in different jobs in our state. Most children were from the already known leprosy-endemic states of Chhattisgarh (25%) and Bihar (23%). Migration has been considered as one of the determining factors in the expansion of leprosy. An intense migration of rural population to urban cities in search of employment leads to dissemination of infection to the cities where this disease had previously been absent or where the number of cases had been minimal.13
Contact history of 23.6% is quite comparable with the figures observed by former studies (21.9%, 29%).19,20 A close, prolonged contact between susceptible healthy individuals and untreated MB cases maintains an epidemiological cycle of leprosy with household transmission being the most significant.21 Rodrigues et al. observed that children with a family history of leprosy had an 8.7-fold higher chance of developing the disease in comparison to those who did not have affected members in the family.22 This risk of contracting leprosy is not restricted to the group of close relatives living under the same roof (household contacts) but also includes neighbourhood and social contacts (extra-household contacts). In a study conducted by Moet et al., 52.6% reported having had contact with another infected individual inside the household and 25% in their social circle. There was a statistically significant difference between the two types of contact according to sex, household contact being more common among girls and social contact more common among boys.17
Many children (40%) had >5 skin lesions, whereas single lesion leprosy was found in 29%. Previous studies have shown variable results, a few observing single lesion and a few finding multiple lesions as the most frequent presentation. Hypopigmented skin lesions were common, involving the extremities, face and trunk akin to the sites reported by Dogra et al.10 and Nair.23
Borderline tuberculoid (BT) leprosy was the most common clinical subtype (49.1%) followed by borderline lepromatous (BL) leprosy (18%), mid-borderline (BB) leprosy (14%) and lepromatous (LL) (9.1%). Similar predominance of tuberculoid leprosy has been observed in earlier studies, conducted across different parts of the country.10,11,15,19,20,23 Peripheral nerve trunk was thickened in 76.4%, and of these, 58.3% had more than one nerve involvement. Most of the child leprosy studies have reported nerve involvement in the range of 40–80% with more than 1 nerve involvement in a good number.10,15,23-25 Peripheral nerve involvement, and that too more than one, in children affected with leprosy is considered as one of the risk factors for developing reactions and deformities.26
Smear positivity was detected in 25.4% of patients and MB case detection rate was 76%. Almost comparable results have been reported in former studies for both these variables.2,10,14,19,27,28 This indicates circulation of Mycobacterium leprae in this younger age group, thereby, maintaining the chain of transmission in the community.
Clinicohistopathological correlation was established in 65.2%. Clinicohistopathological diagnosis is important as it aids in identifying those MB cases which could be misdiagnosed as PB because of their clinical presentation.
Our study revealed lepra reaction rate of 21.8% which is less than that reported by Dogra et al.10 (33.9%) and Jain et al.24 (29.7%). Type one reaction (18.2%) was commoner than type two reaction (3.6%) most likely due to the prepondernace of tuberculoid leprosy in our patients. Neuritis, a risk factor for deformities, was present in 3.5% of cases. Two patients with neuritis had an associated type one reaction: one diagnosed with mid-borderline (BB) leprosy had both type 1 reaction and neuritis at presentation while the second patient with borderline tuberculoid (BT) leprosy had a single thickened nerve that developed neuritis about nine months after starting therapy. The third patient with neuritis had a severe type two reaction at the first visit with erythema nodosum leprosum (ENL), fever and tender lymph nodes.
Grade 2 disability rate (7.3%) in our study was not as high as found in most Indian studies. The possible explanation could be one as majority of the children (56.3%) presented within one year of developing signs and symptoms of disease.
Treatment completion rate in the present study was low (54.4%) while 45.5% dropped out after the first visit or defaulted treatment or were not adequately compliant to the treatment. This may be because most children were not permanent residents and may have gone back to their home towns once they were diagnosed with the disease. On follow-up, no signs of relapse were mentioned in the records although the period of follow-up (varying from six months to two years) was not adequate as per recommendations.
We made an attempt to follow the trend of significant epidemiological and operational parameters by comparing the data of recent decade (2008–2017) with that of previous decade (1998–2007) [Table 3]. Age distribution revealed the occurrence of leprosy even in younger age groups, signifying an early exposure to open cases in their close vicinity. As shown in Table 3, the percentage of MB cases increased from 66% to 88% although the smear positivity declined marginally. This could be attributed to the modification of MB case definition by the WHO considering the number of skin lesions and the number of nerves involved in spite of smear negativity. Reaction rate (Type 1 reaction) was also higher. A significant increase in migrant to local population ratio was noted. On the contrary, an appreciable decline was perceived in Grade 2 disability and defaulter rate, ascribed to the better information, education and counseling (IEC) activities being carried out under National Leprosy Eradication Programme (NLEP) at every level. However, the proportion of child leprosy cases was similar in both decades. Similar slow and slight decline in percentage of child cases has been observed at the both national and global level between 2005 and 2017 [Figure 3].29-41
| Variables | Past decade (1998–2008) | Recent decade (2008–2018) |
|---|---|---|
| Total new cases | 837 | 711 |
| Child cases | 30 | 25 |
| Percentage of child leprosy* | 3.58% | 3.52% |
| M : F ratio | 9:1 | 1:1 |
| Age group (in years) | ||
| 0–5 | - | 4% |
| 6–10 | 26% | 44% |
| 11–15 | 73% | 52% |
| Migrant : Local | 3:1 | 11:1 |
| MB : PB | 2:1 | 6:1 |
| >1 nerve involvement | 56% | 60% |
| SSS positivity (%) | 25.4% | 20% |
| Reactions (T1:T2) | 13.3% (3:1) | 32% (7:1) |
| Grade 2 disability (%) | 13.3% | 4% |
| Defaulter rate (%) | 60% | 36% |
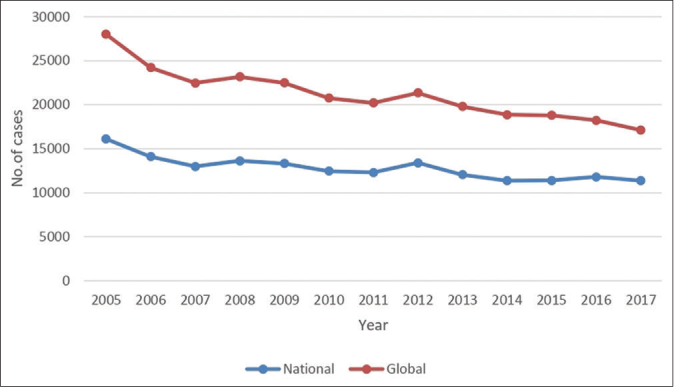
- Trends in new case detection among children at national and global level between 2005 and 2017
Limitations
As it is a hospital-based retrospective study with no active surveys, field studies involving active surveillance are required for corroborating the results obtained. The exact relapse rate could not be calculated as the duration of follow-up was not adequate.
Conclusion
Since no significant decline in child leprosy cases has been witnessed over two decades, it means that active transmission of infection in the community is still on the rise and stakeholders should not let their guard down. Periodic screening of contacts, especially children, is a must to interrupt this transmission. An issue of increase in the number of migrant cases in low endemic areas should be addressed seriously by government organizations. To accomplish this, an essential triad of disease control, that is, early case detection, timely treatment and breaking the chain of transmission, needs to be strengthened and covered under one umbrella, that is, national programme. This warrants an active participation of both governmental and non-governmental organizations to work in full coordination with high level of motivation to root out leprosy and its associated complications, affecting both the physical and mental health of the younger generation at the national and global levels.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Global Leprosy Strategy 2016-2020: Accelerating towards a Leprosy Free World, WHOs SEARO/Department of control of Neglected Tropical Diseases, New Delhi. 2016.
- [Google Scholar]
- Global leprosy update, 2018: Moving towards a leprosy free world. Wkly Epidemiol Rec. 2019;94:389-412.
- [Google Scholar]
- WHO Expert Committee on Leprosy, Seventh Report, WHO Technical Report Series No. 874 Geneva: World Health Organization; 1998.
- [Google Scholar]
- Classification of leprosy according to immunity. A five group system. Int J Lepr Other Mycobact Dis. 1966;34:255-73.
- [Google Scholar]
- Clinical, histopathological and immunological features of the five type classification approved by the Indian association of leprologists. Lepr India. 1982;54:22-32.
- [Google Scholar]
- WHO Expert Committee on Leprosy, Sixth Report, WHO Technical Report Series No. 847 Geneva: World Health Organisation; 1988.
- [Google Scholar]
- Disease Classification P. No. 17. Ch. 5. 2019. NLEP. Available from: http://www.nlep.nic.in/training.html [Last accessed on 2019 Nov 09]
- [Google Scholar]
- National Sample Survvey Office. 2010. Ministry of Statistics and Programme Implementation, NSS Report No. 533, Government of India. Available from: http://www.mospi.nic.in/data.html [Last accessed on 2021 Mar 25]
- [Google Scholar]
- NLEP-Progress Report for the Year 2017-18 Ending on 31st March 2018. 2018. Central Leprosy Division, Directorate General of Health Services. New Delhi: Government of India; Available from: http://www.nlep.nic.in/data.html [Last accessed on 2019 Sep 05]
- [Google Scholar]
- Childhood leprosy through the post-leprosy-elimination era: A retrospective analysis of epidemiological and clinical characteristics of disease over eleven years from a tertiary care hospital in North India. Lepr Rev. 2014;85:296-310.
- [CrossRef] [PubMed] [Google Scholar]
- Childhood leprosy in the post elimination era: A vision achieved or a concern growing at large. Indian J Paediatr Dermatol. 2018;19:26-30.
- [CrossRef] [Google Scholar]
- Perfil clõânico-epidemioloâgico da hansenõâase em menores de quinze anos no municõâpio de juazeiro-BA. Rev Bras promocë sauâde. 2013;26:208-15. Available from: http://www.periodicos.unifor.br/rbps/article/view/2906 [Last accessed on 2019 Sep 10]
- [CrossRef] [Google Scholar]
- Leprosy in children and adolescents under 15 years old in an urban centre in Brazil. Mem Inst Oswaldo Cruz. 2016;111:359-64.
- [CrossRef] [PubMed] [Google Scholar]
- The burden of leprosy in children and adolescents in rural Southern Ethiopia. Paediatr Int Child Health. 2014;34:24-8.
- [CrossRef] [PubMed] [Google Scholar]
- Current scenario of childhood leprosy at a tertiary care hospital in Southern Rajasthan. Indian Dermatol Online J. 2017;8:494-5.
- [CrossRef] [PubMed] [Google Scholar]
- Post-elimination status of childhood leprosy: Report from a tertiary care hospital in South India. Biomed Res Int. 2013;2013:328673.
- [CrossRef] [PubMed] [Google Scholar]
- Physical distance, genetic relationship, age, and leprosy classification are independent risk factors for leprosy in contacts of patients with leprosy. J Infect Dis. 2006;193:346-53.
- [CrossRef] [PubMed] [Google Scholar]
- A retrospective study of the epidemiology of leprosy in Cebu: An eleven-year profile. PLoS Negl Trop Dis. 2013;7:e2444.
- [CrossRef] [PubMed] [Google Scholar]
- An epidemiologic study of childhood leprosy from Delhi. Pediatr Dermatol. 2005;22:489-90.
- [CrossRef] [PubMed] [Google Scholar]
- A study of leprosy in children, from a tertiary paediatric hospital in India. Lepr Rev. 2006;77:160-2.
- [CrossRef] [PubMed] [Google Scholar]
- Susceptibility to leprosy is associated with PARK2 and PACRG. Nature. 2004;427:636-40.
- [CrossRef] [PubMed] [Google Scholar]
- Factors associated with leprosy in children contacts of notified adults in an endemic region of Midwest Brazil. J Pediatr (Rio J). 2020;96:593-9.
- [CrossRef] [PubMed] [Google Scholar]
- A clinico-epidemiological study of pediatric leprosy in the urban leprosy centre of a tertiary care institute. Indian J Paediatr Dermatol. 2017;18:24-7.
- [CrossRef] [Google Scholar]
- Childhood leprosy in an urban clinic, Hyderabad, India: Clinical presentation and the role of household contacts. Lepr Rev. 2002;73:248-53.
- [CrossRef] [PubMed] [Google Scholar]
- Understanding the Type 1 reactional state for early diagnosis and treatment: A way to avoid disability in leprosy. An Bras Dermatol. 2013;88:787-92.
- [CrossRef] [PubMed] [Google Scholar]
- Childhood leprosy: Profiles from a leprosy referral hospital in West Bengal, India. Indian J Lepr. 2010;82:33-7.
- [Google Scholar]
- Childhood leprosy: A retrospective study. J Public Health Epidemiol. 2010;2:267-71.
- [Google Scholar]
- Global leprosy situation, 2006. Wkly Epidemiol Rec. 2006;81:309-16. Available from: http://www.who.int/wer [Last accessed on 2019 Dec 24]
- [Google Scholar]
- Global leprosy situation, 2007. Wkly Epidemiol Rec. 2007;82:225-32. Available from: http://www.who.int/wer [Last accessed on 2019 Dec 24]
- [Google Scholar]
- Global leprosy situation, beginning of 2008. Wkly Epidemiol Rec. 2008;83:293-300. Available from: http://www.who.int/wer [Last accessed on 2019 Dec 24]
- [Google Scholar]
- Global leprosy situation, 2009. Wkly Epidemiol Rec. 2009;84:333-40. Available from: http://www.who.int/wer [Last accessed on 2019 Dec 24]
- [Google Scholar]
- Global leprosy situation, 2010. Wkly Epidemiol Rec. 2010;85:337-48. Available from: http://www.who.int/wer [Last accessed on 2019 Dec 24]
- [Google Scholar]
- Leprosy update 2011. Wkly Epidemiol Rec. 2011;86:389-400. Available from: http://www.who.int/wer [Last accessed on 2019 Dec 24]
- [Google Scholar]
- Global leprosy situation, 2012. Wkly Epidemiol Rec. 2012;87:317-28. Available from: http://www.who.int/wer [Last accessed on 2019 Dec 24]
- [Google Scholar]
- Global leprosy update, 2012. Wkly Epidemiol Rec. 2012;88:365-78. Available from: http://www.who.int/wer [Last accessed on 2019 Dec 24]
- [Google Scholar]
- Global leprosy update, 2013. Wkly Epidemiol Rec. 2013;89:389-400. Available from: http://www.who.int/wer [Last accessed on 2019 Dec 24]
- [Google Scholar]
- Global leprosy update, 2014. Wkly Epidemiol Rec. 2014;90:461-73. Available from: http://www.who.int/wer [Last accessed on 2019 Dec 24]
- [Google Scholar]
- Global leprosy update, 2015. Wkly Epidemiol Rec. 2015;91:405-20. Available from: http://www.who.int/wer [Last accessed on 2019 Dec 24]
- [Google Scholar]
- Global leprosy update, 2016. Wkly Epidemiol Rec. 2016;92:501-20. Available from: http://www.who.int/wer [Last accessed on 2019 Dec 24]
- [Google Scholar]
- Global leprosy update, 2017. Wkly Epidemiol Rec. 2017;93:444-56. Available from: http://www.who.int/wer [Last accessed on 2019 Dec 24]
- [Google Scholar]






