Translate this page into:
Clinical and hormonal evaluation in facial pigmentary demarcation lines: A pilot study
Correspondence Address:
Sushruta Kathuria
Department of Dermatology and Venereology, All India Institute of Medical Sciences, New Delhi-110 029
India
| How to cite this article: Kathuria S, Khunger N, Ramesh V. Clinical and hormonal evaluation in facial pigmentary demarcation lines: A pilot study. Indian J Dermatol Venereol Leprol 2012;78:742-744 |
Sir,
Facial pigmentary demarcation lines (PDL) were described by Malakar and Dhar in Indian population [1] and are classified into 3 types F, G, and H. [2] Increased incidence of PDL in females and type B PDL in pregnancy suggests that increased female sex hormones may cause PDL. [3] Hence, a cross-sectional study was conducted on consecutive patients of facial PDL to evaluate the hormonal profile. Patients with facial PDL presenting to the authors in the Dermatology Outpatient Department of Safdarjung Hospital from July 2009 to January 2010 were recruited. PDL was diagnosed clinically by two authors independently and was classified as: F: ′V′ shape on lateral aspect of face between malar prominence and temple, G: ′W′ shape on lateral aspect of face between malar prominence and temple, and H: Linear band of hyperpigmentation from angle of mouth to lateral aspect of chin. Blood sample (5 ml) was taken after informed consent for serum levels of estradiol, progesterone, follicle stimulating hormone (FSH), leutinizing hormone (LH), and prolactin on 4 th day of menstrual cycle. Twenty-seven patients (26 females, 1 male) were recruited, out of which 18 got hormonal levels done. The demographic and clinical details are summarized in [Table - 1]. The mean age of onset was 24.4 years (10-50 years), and mean duration of the disease was 4.89 years (2 months to 22 years). One was a field worker, 18 (66.6%) had indoor job, and 8 (29.6%) were housewives with average 1.4 hours sun exposure daily. Sunscreen was regularly used by 12 (44.4%) and physical barriers of photoprotection by 10 (37%). Family history of facial PDL was present in 6 (22.2%). Menstrual cycle was regular in 20 (74%), irregular in 4 (14.8%), and menopause was present in 1 (3.7%). None had used oral contraceptive pills or any other hormonal drugs. Two had onset of PDL in first pregnancy. Nine patients had taken prior treatment such as hydroquinone, steroids, and retinoids with minimal response in 3.
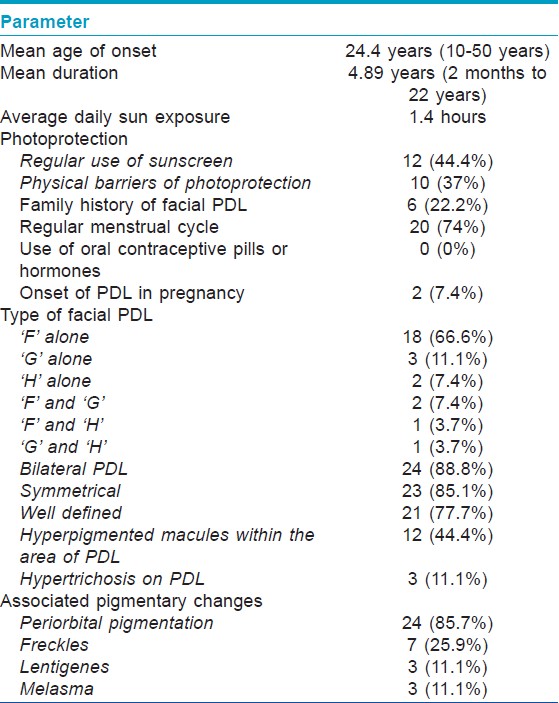
The commonest type of facial PDL was ′F′ in 21 [Figure - 1], G in 6 [Figure - 2], H in 4 [Figure - 3]. Eighteen (66.6%) had ′F′ alone, 3 (11.1%) had ′G′ alone, 2 (7.4%) had ′H′ alone, 2 (7.4%) had coexistent ′F′ and ′G,′ and 1 each had coexistent ′F′ and ′H′ and ′G′ and ′H.′ We noted abrupt horizontal line of demarcation between hyperpigmented and normally pigmented area over forehead associated with other PDL in 10 (37%) patients [Figure - 4]. PDL was bilateral in 24 (88.8%), symmetrical in 23 (85.1%) and well defined in 21 (77.7%). Hyperpigmented macules within the area of PDL [Figure - 5] was present in 12 (44.4%), and hypertrichosis present on the PDL was seen in 3 (11.1%). Periorbital pigmentation was present in 24 (85.7%), freckles in 7 (25.9%), lentigenes in 3 (11.1%), melasma in 3 (11.1%), and PDL on bilateral arms in 1.
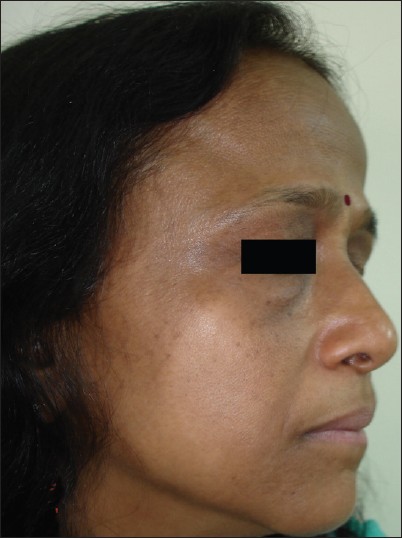 |
| Figure 1: Pigmentary demarcation line type 'F.' |
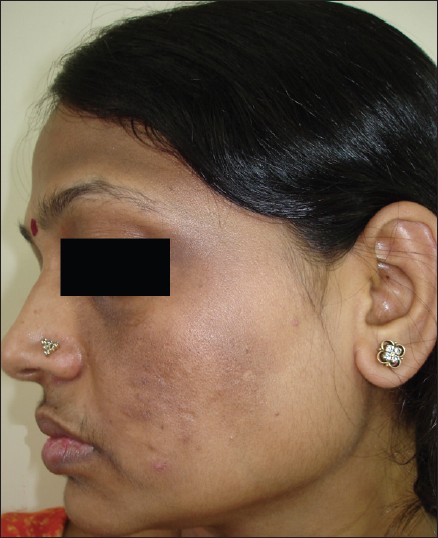 |
| Figure 2: Pigmentary demarcation line type 'G.' |
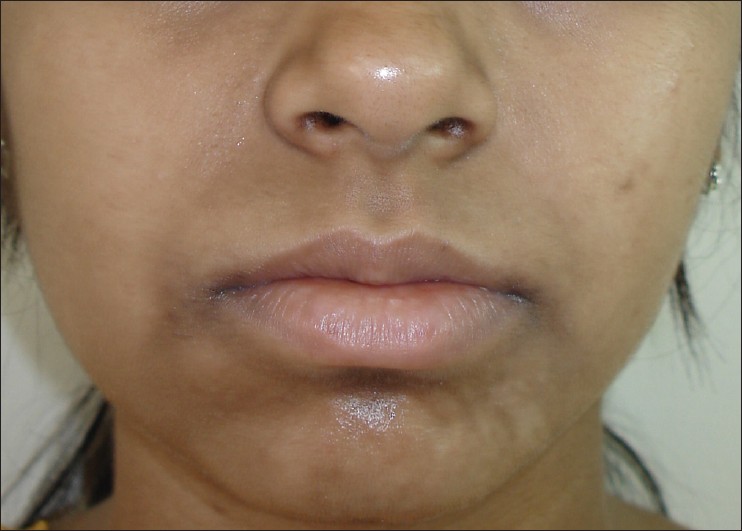 |
| Figure 3: Pigmentary demarcation line type 'H.' |
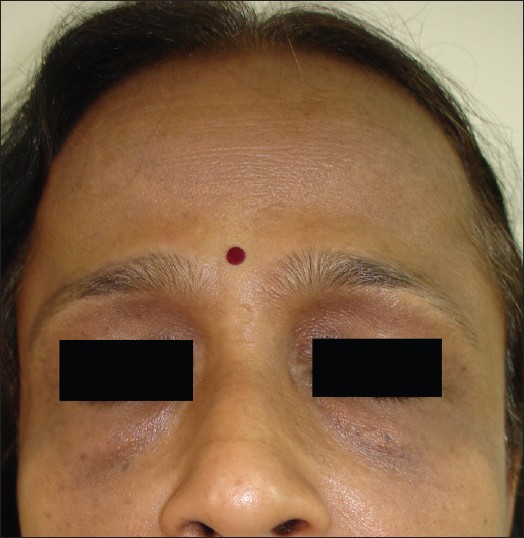 |
| Figure 4: Proposed Pigmentary demarcation line type 'I.' |
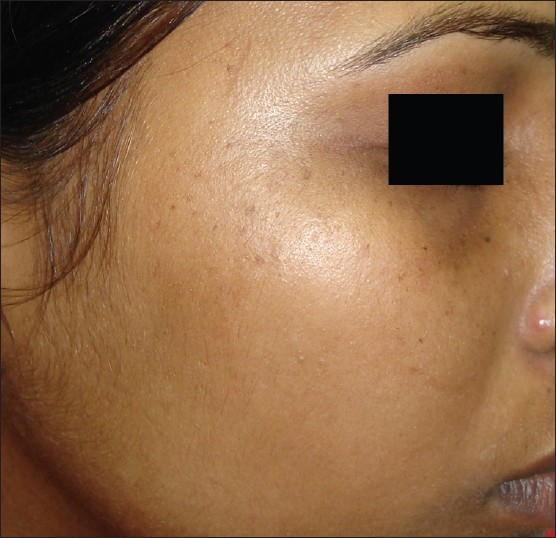 |
| Figure 5: Speckling in Pigmentary demarcation line |
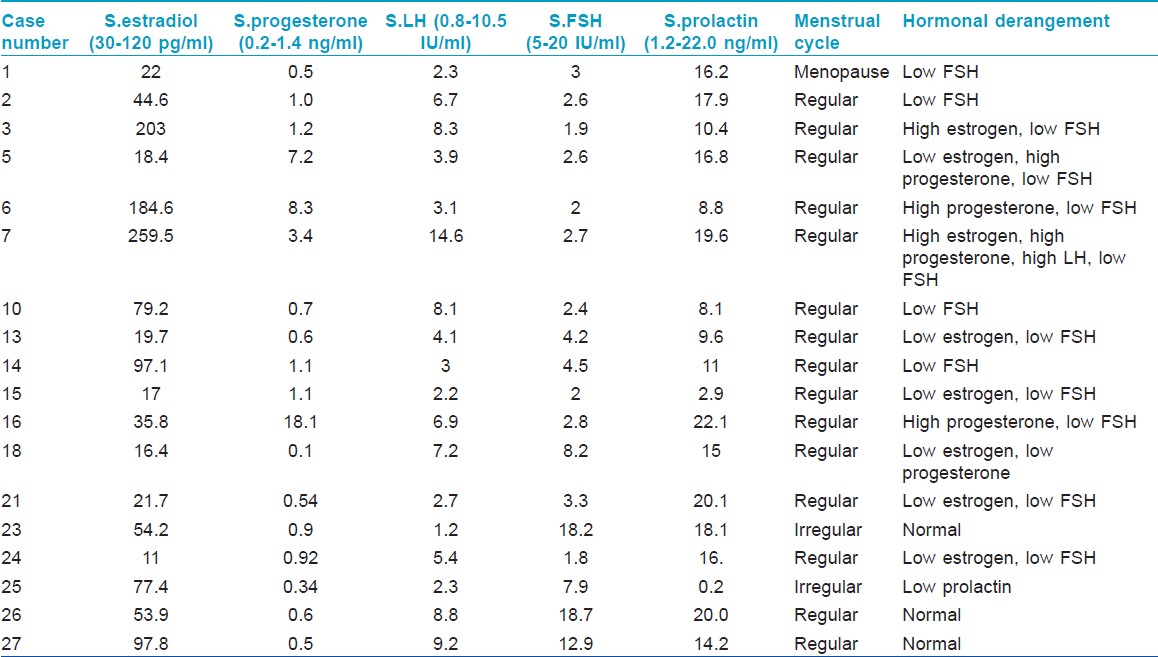
Hormonal levels were abnormal in 15/18 (83.3%) patients [Table - 2], of which, all but one had regular menstrual cycles. Low FSH was seen in 13 (72.2%), low estrogen in 6 (33.3%), high progesterone in 4 (22.2%), high estrogen in 2 (11.1%), low progesterone in 1 (5.5%), high LH in 1 (5.5%), and low prolactin in 1 (5.5%). Lesional biopsy conducted on 4 consenting patients showed basal and suprabasal pigmentation with mild pigment incontinence in papillary dermis.
Higher female preponderance in this study maybe due to increased cosmetic awareness in females. The onset of facial PDL is in puberty, [2] but in our study, onset was in young adulthood and onset in pregnancy was in only 7.4%, diminishing the role of increased female sex hormones as a cause of PDL.
More than three-fourth had abnormal hormonal levels, low FSH, and low estrogen being the commonest. Levels of sex hormones have not been reported previously in facial PDL. However, a single case report of amenorrheic Chinese woman documents low estradiol and FSH with type B PDL. [4] It is difficult to correlate the abnormal hormonal profile with pathogenesis of facial PDL in this study as one does not know if abnormal hormonal levels preceded the onset of PDL. The heterogeneous nature of hormonal levels further complicates the interpretation. However, this study does show that female sex hormones are low and not increased in facial PDL.
Family history of PDL in 22.2% suggests strong genetic predisposition. Bilaterality, symmetry, definition, and speckling are important morphological features of PDL. The speckling (hyperpigmented macules) is different from lentigenes as they remain confined to the hyperpigmented area of PDL. Periorbital melanosis is considered as an extension of type ′F′ PDL, [5] but we observed it with all types of PDL. A horizontal line of demarcation between hyperpigmented and normally pigmented skin suggestive of PDL was noted on the forehead in 37% patients, and we propose that it should be considered separately as type ′I′ PDL.
The limitations of this study were small number of patients, lack of control group to compare hormonal changes in other pigmentary diseases and normal population, lack of follow up and that patients did not give free consent for performing biopsies. It is proposed that more studies should be conducted on larger group of patients with recent onset of facial PDL so that a temporal correlation between hormonal abnormalities and pathogenesis of PDL can be ascertained.
| 1. |
Malakar S, Dhar S. Pigmentary demarcation lines over the face. Dermatol 2000;200:85-6.
[Google Scholar]
|
| 2. |
Somani VK, Razvi F, Sita VNVL. Pigmentary demarcation lines over the face. Indian J Dermatol Venereol Leprol 2004;70:336-41.
[Google Scholar]
|
| 3. |
Grimes P, Nordlund JJ, Pandya AG, Taylor S, Rendon M, Ortonne JP. Increasing our understanding of pigmentary disorders. J Am Acad Dermatol 2006;54:S255-61.
[Google Scholar]
|
| 4. |
Ma HJ, Zhao G, Dang YP. Type B pigmentary demarcation lines in a Chinese amenorrheic woman. Int J Dermatol 2008;47:505-7.
[Google Scholar]
|
| 5. |
Malakar S. Lahiri K, Banerjee U, Mondal S, Sarangi S. Periorbital melanosis is an extension of pigmentary demarcation line-F on face. Indian J Dermatol Venereol Leprol 2007;73:323-5.
[Google Scholar]
|
Fulltext Views
12,490
PDF downloads
3,522





