Translate this page into:
Clinical and immunological predictors of post-rituximab paradoxical pemphigus flare: A prospective cohort study
Corresponding author: Dr. Vishal Gupta, Department of Dermatology and Venereology, All India Institute of Medical Sciences, New Delhi, India, doctor.vishalgupta@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Gupta V, Ahuja R, Sindhuja T, Imran S, Viswanathan GK, Tembhre MK, et al. Clinical and immunological predictors of post-rituximab paradoxical pemphigus flare: A prospective cohort study. Indian J Dermatol Venereol Leprol. 2025;91:3-8. doi: 10.25259/IJDVL_894_2023
Abstract
Background
Paradoxical flare of pemphigus following rituximab infusion has been reported previously, however, its incidence or risk factors have not been studied in detail.
Objectives
To evaluate the clinical and immunological predictors associated with post-rituximab paradoxical pemphigus flare.
Materials and Methods
This was a prospective cohort study including adult patients with pemphigus vulgaris or foliaceus who were treated with rituximab. Patients were administered 1000 mg of intravenous rituximab on days 0 and 14 (Rheumatoid arthritis (RA) protocol), with or without oral prednisolone and/or conventional immunosuppressive agents. Baseline clinical and immunological predictors of post-rituximab pemphigus flares were assessed.
Results
Fifty patients (mean age 40.44 ± 12.36 years) with a mean pemphigus disease area index (PDAI) score of 27.8 ± 15.48 were administered rituximab. Post-rituximab flare occurred in 10 (20%) patients after a mean of 14.1 ± 4.33 days after the first rituximab infusion. The mean baseline PDAI score (36.4 ± 11.7 vs. 25.6 ± 15.7, P = 0.02) and serum anti-Dsg1 levels (1216.8 ± 850.1 vs. 592 ± 562.12 RU/mL, P = 0.03) were statistically significantly higher in patients experiencing a flare. Using ROC-curve analysis, a PDAI score of ≥28 (OR 8.3, 95% CI 1.5–44.7) was 80% sensitive and 67.5% specific in predicting post-rituximab flare, while serum anti-Dsg1 level of ≥1137.78 RU/ml had a sensitivity of 60% and specificity of 85%.
There was no significant difference in terms of affected body surface area, type of pemphigus, starting prednisolone dose, oral immunosuppressive adjuvant, serum anti-Dsg3, serum anti-AchRM3, and peripheral CD19+ B cell population.
Limitations
Our study is limited by a relatively small sample size. Immunological factors were not evaluated at the time of pemphigus flare. Though these unexpected pemphigus flares are likely to be associated with rituximab infusion, the possibility of spontaneous disease exacerbation cannot be entirely excluded.
Conclusions
Patients with more severe pemphigus or high serum anti-Dsg1 are at risk of post-rituximab paradoxical flare, and may benefit from rituximab administration under close monitoring.
Keywords
pemphigus
paradoxical flare
rituximab
PDAI
predictors
Introduction
Pemphigus is a rare, potentially fatal autoimmune bullous dermatosis involving the skin and/or mucosae with autoantibodies targeting desmosomal transmembrane proteins. Over the last few years, rituximab has emerged as an effective first-line therapy for both pemphigus vulgaris and foliaceus.1 RITUX-3 trial has established the efficacy of rituximab; 89% of patients assigned to the short-term prednisolone and rituximab arm attained complete remission off therapy compared to 34% in the prednisolone-only arm.2 With its increasing use, a few reports of a paradoxical flare of pemphigus following rituximab infusion have emerged in the literature,3–8 however, its exact incidence or risk factors have not been studied in detail.
The present study aimed to evaluate the baseline clinical and immunological factors associated with post-rituximab paradoxical pemphigus flare, and to identify a subgroup of patients who might require closer monitoring in the immediate post-rituximab infusion period.
Materials and Methods
Study design and participants
This was a prospective observational cohort study conducted at the All India Institute of Medical Sciences (AIIMS), New Delhi, India, from August 2021 to October 2022. The protocol was registered (CTRI/2020/10/028675) and approved by the Institute Ethics Committee. Adult (≥18 years) patients with histologically confirmed pemphigus vulgaris or foliaceus with active lesions who were planned for rituximab infusion (rheumatoid arthritis (RA) protocol) were recruited after informed consent and followed up at 2 and 4 weeks after the first rituximab dose. Patients with inactive pemphigus, those who had received rituximab in the last 12 months, or had other concomitant autoimmune diseases were excluded.
Post-rituximab pemphigus flare
There is currently no consensus on the definition of post-rituximab pemphigus flare. For this study, we defined it as a disease exacerbation requiring an increase in prednisolone dose (≥10 mg/day) or administration of a dexamethasone pulse or IVIG infusion at any time in the four weeks after the first rituximab infusion.
Clinical and immunological markers
A detailed history and clinical examination were performed at baseline. Pre-rituximab pemphigus severity was assessed using the Pemphigus Disease Area Index (PDAI) score and by estimating the affected body surface area, on the day of rituximab injection before infusion. Oral mucosal disease severity was assessed using the Pemphigus Oral Lesions Intensity Score (POLIS) as well. PDAI is a validated scoring system that takes into account the number and size of lesions and their anatomical location. The score ranges from 0 to 250 points (120 points for skin activity, 10 for scalp involvement, and 120 for mucosal disease).9 A PDAI score of 0–14, 15–44, and >45 signifies mild/moderate, significant, and extensive pemphigus disease, respectively.10 POLIS is a recently validated 9-item tool for quantifying oral mucosal disease severity in pemphigus vulgaris. Six items assess the patient-perceived symptoms and quality of life impairment secondary to mucosal lesions, while the other three items address the clinical disease severity by accounting for the number of oral mucosal sites, and the size and depth of erosions. POLIS score ranges from 0 to 36.11
A venous blood sample was collected for the evaluation of anti-Dsg and anti-AchRM3 antibodies, and B-cell population. Anti-Dsg1 and anti-Dsg3 antibody levels (Euroimmun Medizinische Labordiagnostika AG, assay range 2–200 RU/mL), and anti-AchRM3 (FineTest, Wuhan Fine BioTech Co Ltd, assay range 3.125–200 ng/mL) antibody levels were estimated by using commercially available enzyme linked immunosorbent assay (ELISA) kit as per manufacturers’ instructions. Samples with results above the reference range were re-tested after adequate dilution. The total CD19+ B-cell count in the peripheral blood was assessed by flow cytometry. The lymphocytes were stained with fluorescein isothiocyanate–labelled anti-human CD45, phycoerythrin-labelled anti-human CD27 antibodies, and allophycocyanin-labelled anti-human CD19 antibodies as per the manufacturer’s protocol.
The study flowchart is shown in Figure 1.
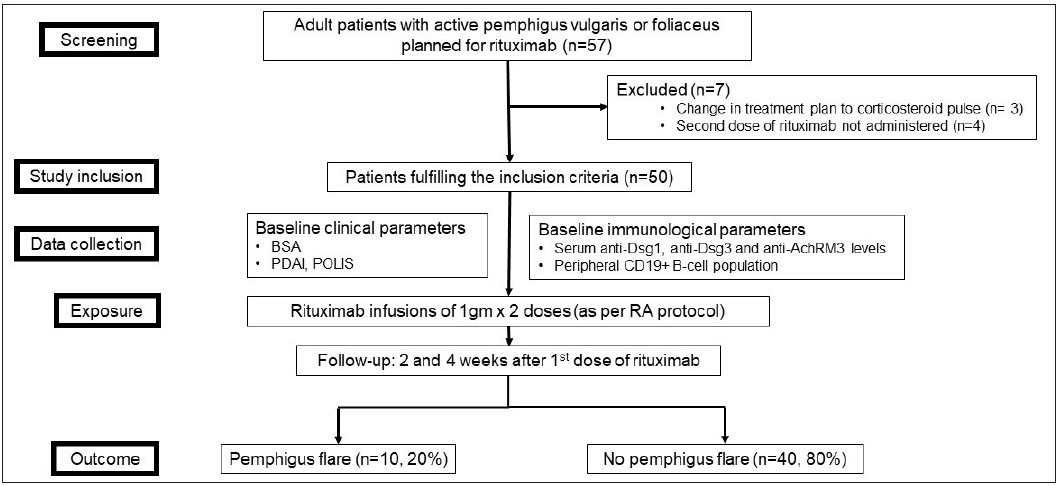
- Study flowchart. (BSA: body surface area; PDAI: pemphigus disease area index; POLIS: pemphigus oral lesions intensity score; RA: rheumatoid arthritis)
Statistical analysis
We compared the baseline clinical and immunological parameters between patients who developed a flare post-rituximab infusion and those who did not. The following variables were selected a priori to be evaluated as predictors of flare: PDAI (total and mucosal) score, POLIS, affected body surface area, pemphigus type, disease duration, starting prednisolone dose, oral immunosuppressive adjuvant used or not, serum anti-Dsg1 and anti-Dsg3 antibody levels, anti-AchRM3 antibody levels and CD19+ B-cell population. Continuous and categorical variables were compared using the Mann–Whitney U test and Chi-square test, respectively. A p-value of <0.05 was considered statistically significant. Statistical analysis was performed using Stata 16 software (StataCorp. 2019. College Station, TX: StataCorp LLC).
For parameters found to have a statistically significant difference, a receiver operating characteristic (ROC) curve was calculated to determine cut-off values to predict flare with optimal sensitivity, specificity, and positive and negative predictive values.
Results
Baseline characteristics and clinical course
Of the 57 patients with pemphigus planned for rituximab RA protocol during the study period, 50 patients (mean age 40.44 ± 12.36 years; 24 [48%] males and 26 [52%] females) were included. Seven patients were excluded because the treatment plan was changed from rituximab to corticosteroid pulses (n = 3) or they were not administered the second dose of rituximab (n = 4). Most of the patients (92%, n = 46) had received systemic treatment earlier, such as oral (n = 40, 80%) and/or pulsed (n = 9, 18%) steroids, conventional oral immunosuppressive agents (n = 36, 72%) and/or rituximab (n = 14, 28%). Of the 50 patients who received rituximab in the current study, four were treatment-naïve, while others had an inadequate response to earlier treatment or had relapsed.
Out of the 50 included patients, 47 had pemphigus vulgaris and three had pemphigus foliaceus. The mean PDAI score was 27.8 ± 15.48 (range 2–71). Ten (20%) patients had moderate disease (PDAI <15), 33 (66%) had severe disease (PDAI 15–44) and 7 (14%) had extensive disease (PDAI >45). Besides rituximab infusion, 47 patients received other concomitant treatment; 18 (36%) patients received oral prednisolone along with conventional immunosuppressive agents, 25 (50%) received only oral prednisolone and 4 (8%) received only conventional immunosuppressive agents. Three (6%) patients received rituximab monotherapy. Ten (20%, 95% CI 11–33%) patients (all with pemphigus vulgaris) developed a post-rituximab disease flare, corresponding to a mean increase in PDAI score of 11.8 ± 11.07 (range 3–35) points. Eight out of these 10 patients developed an exacerbation after the first rituximab infusion, while the remaining 2 patients flared after the second infusion. The mean time to flare was 14.1 ± 4.33 days (range 6–22 days) after the first rituximab infusion. The flare was treated by a hike in daily prednisolone dose in all patients (mean increase of 15 ± 5.27 mg/d, range 10–20 mg). Additionally, one patient was treated with a dexamethasone pulse (100 mg for 3 consecutive days) and initiated mycophenolate mofetil. One patient with pemphigus vulgaris who developed a severe disease flare (PDAI score at baseline 46; on day 6 after the first rituximab infusion, 81) died due to septic shock. The second rituximab infusion was given as per schedule to all the other patients, except one, for whom it was postponed by one week.
Correlation between clinical disease severity scores and serological parameters
The mucosal PDAI score and POLIS correlated strongly (r = 0.86, P < 0.001). A moderate correlation was found between PDAI and anti-Dsg1 levels (r = 0.49, P < 0.001); PDAI and anti-Dsg3 levels (r = 0.42, P = 0.002), and between POLIS and anti-Dsg3 levels (r = 0.54, P < 0.001), while a strong correlation was present between mucosal PDAI and anti-Dsg3 levels (r = 0.63, P < 0.001). There was no significant correlation between anti-AchRM3 levels and PDAI (r = 0.19, P = 0.19), mucosal PDAI (r = 0.14, P = 0.33) or POLIS (r = 0.06, P = 0.69).
Comparative baseline clinical and immunological parameters
The mean PDAI score just before initiation of rituximab was statistically significantly higher in patients who experienced a post-rituximab flare (36.4 ± 11.7 [range 22–60] vs. 25.6 ± 15.7 [range 2–71], P = 0.02). Mean affected body surface area was higher in patients who flared compared to those who did not (4.4 ± 2.8% [range 1–11%] vs. 3.2 ± 3% [range 1–18%]), showing a trend toward statistical significance (P = 0.06). There was no statistically significant difference between patients who flared and those who did not in terms of mean age (43 ± 15 vs. 39.8 ± 11.7 years, P = 0.47), mean duration of illness (46.4 ± 63.6 vs. 34.7 ± 34.3 months, P = 0.88), type of pemphigus (10/47 [21.2%] pemphigus vulgaris vs. 0/3 [0%] pemphigus foliaceus flared, P = 1.00), use of oral prednisolone (9 [90%] vs. 31 [77.5%], P = 0.66), mean starting prednisolone dose (30.5 ± 7.6 mg/d vs. 23.5 ± 14.7 mg/d, P = 0.47) and use of oral immunosuppressive adjuvant (4 [40%] vs. 18 [45%], P = 1.00). Mucosal PDAI (10.2 ± 10.9 vs 9.2 ± 7.4, P = 0.94) and POLIS (13.8 ± 13.3 vs. 10.7 ± 8.2, P=0.61) also did not differ statistically significantly between the two groups.
The mean serum anti-Dsg1 levels were statistically significantly higher in patients who experienced a post-rituximab flare (1216.8 ± 850.1 vs. 592 ± 562.12 RU/mL, P = 0.03). However, the difference in serum anti-Dsg3 (1105.8 ± 969.8 vs. 619.3 ± 593.1 RU/mL, P = 0.20) and serum anti-AchRM3 (92.6 ± 182.2 vs. 70.4 ± 135.3 ng/mL, P = 0.80) antibody levels were statistically not significant between the two groups. Similarly, there was no statistically significant difference in the CD19+ B cell count (2276.0 ± 4293.0 vs. 1339.0 ± 1398.6 cells/mm3, P = 0.87) or percentage (12.3 ± 9.4% vs. 11.4 ± 7.7%, P = 0.85) between both groups.
Table 1 summarises the comparison of clinical and immunological parameters associated with the post-rituximab pemphigus flare.
| Post-rituximab flare (n = 10) | No flare (n = 40) | P value | |
|---|---|---|---|
| Clinical factors | |||
| Mean age (years) | 43 ± 15 (25–67) | 39.8 ± 11.7 (18–66) | 0.47 |
| Mean duration of illness (months) | 46.4 ± 63.65 (3–216) | 34.75 ± 34.3 (2–120) | 0.88 |
| Pemphigus type | 1.00 | ||
| • Vulgaris | 10 | 37 | |
| • Foliaceus | 0 | 3 | |
| Oral prednisolone used | 9 (90%) | 31 (77.5%) | 0.66 |
| Mean starting prednisolone dose (mg/d) | 30.5 ± 7.6 (20–40) | 23.5 ± 14.7 (0–40) | 0.47 |
| Oral immunosuppressive adjuvant used | 4 (40%) | 18 (45%) | 1.00 |
| Mean affected body surface area (%) | 4.4 ± 2.8 (1–11) | 3.2% ± 3 (1–18) | 0.06 |
| Affected body surface area | 0.06 | ||
| <5% | 6 | 35 | |
| 5–15% | 4 | 4 | |
| >15% | 0 | 1 | |
| Mean PDAI score | 36.4 ± 11.7 (22–60) | 25.6 ± 15.7 (2–71) | 0.02* |
| Mean mucosal PDAI score | 10.2 ± 10.9 (0–28) | 9.2 ± 7.4 (0–25) | 0.79 |
| Mean POLIS score | 13.8 ± 13.3 (0–34) | 10.7 ± 8.2(0–25) | 0.61 |
| Immunological factors | |||
| Mean anti-Dsg1 (RU/mL) | 1216.8 ± 850.1 (31.2–2697.3) | 592 ± 562.12 (0–2205.3) | 0.03* |
| Mean anti-Dsg3 (RU/mL) | 1105.8 ± 969.8 (0–3070.7) | 619.3 ± 593.1 (0–2533.3) | 0.20 |
| Mean anti-AchRM3 (ng/mL) | 92.6 ± 182.2 (1.0–514.5) | 70.4 ± 135.3 (0.4–515.0) | 0.80 |
| CD19+ B cell (%) | 12.3 ± 9.4 (0.4–25.6) | 11.4 ± 7.71 (0.1–30.2) | 0.85 |
| CD19+ B cell (/mm3) | 2276.0 ± 4293.0 (7–13490) | 1339.0 ± 1398.6 (5–5351) | 0.87 |
Predictor of post-rituximab pemphigus flare
A ROC curve was performed to determine the PDAI score and serum anti-Dsg1 cut-off for the prediction of post-rituximab pemphigus flare. The value that had the highest sum of specificity and sensitivity was selected as the optimum cut-off value [Figure 2]. The baseline PDAI cut-off score of ≥28 (OR 8.3, 95% CI 1.5–44.7) was 80% sensitive and 67.5% specific in predicting post-rituximab flare, with a positive predictive value of 38.1% (95% CI 18.1–61.6%) and a negative predictive value of 93.1% (95% CI 77.2–99.2%).
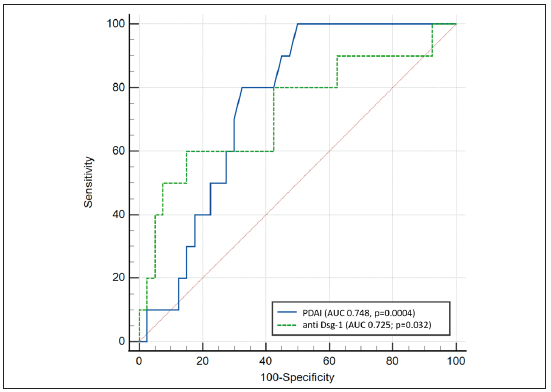
- Receiver operating characteristic curve to determine the optimal baseline pemphigus disease area index (PDAI) score and serum anti-Dsg1 level cut-off value for prediction of post-rituximab pemphigus flare.
The serum anti-Dsg1 level of >1137.78 RU/mL had a sensitivity of 60% and a specificity of 85% in predicting post-rituximab flare, with a positive predictive value of 50% (95% CI 28.5–70.43%) and a negative predictive value of 89.5% (95% CI 79.6–94.8%).
This set of 2 criteria, i.e., baseline PDAI score >28 and anti-Dsg1 levels >1137 RU/mL, provided a sensitivity of 50%, specificity of 87.5%, positive predictive value of 50% (95% CI 18.7–81.3%) and negative predictive value of 87.5% (73.2– 95.8%).
Discussion
With the increasing use of rituximab in pemphigus, some reports of a paradoxical pemphigus flare have emerged in the literature. Paradoxical post-rituximab worsening of pemphigus has been reported at a variable rate of 1.12–47% in earlier retrospective studies.3,5,7,8 Our prospective study puts the incidence of post-rituximab flare at 20%. These flares, apart from potentially interfering with the rituximab schedule, add to disease morbidity and the cost of treatment.
There is no consensus on the definition of post-rituximab pemphigus flare. One study defined it as a >10-point increase in the PDAI score persisting for >2 weeks within the first three months after rituximab administration.4 However, a minimal clinically important difference in the PDAI scores has not been estimated, and therefore a criterion of disease exacerbation necessitating an increase in treatment might be more clinically meaningful as proposed in this study.
The flare in pemphigus activity shortly following rituximab infusion is surprising and could be attributed to other factors as well, primarily spontaneous disease exacerbation. However, certain points suggest a plausible causative link between these flares and rituximab infusion. This phenomenon has been documented by others as well.3–8 There is a strong temporal correlation, with most of the flares happening shortly after the first rituximab dose. Consistent with the previous reports, the majority of our patients (80%) experienced a disease exacerbation after the first dose of rituximab (mean time of 14 days). Upon reviewing the available records of our patients that exacerbated (n = 7), the disease course was found to be largely stable in the days preceding rituximab administration (mean PDAI at a mean of 7 days before rituximab and on the day of its infusion was 33.83 ± 9.31 and 33.33 ± 8.47, respectively). The post-rituximab flare occurred despite continuing the same prednisolone dose (mean 30 mg/day) and other therapies as earlier. Such pemphigus exacerbations have not been described with other treatments. Finally, such a paradoxical exacerbation following rituximab has been reported in other diseases as well, including bullous pemphigoid, neuromyelitis optica, granulomatosis with polyangiitis, Waldenstrom’s macroglobulinemia and cryoglobulinemic vasculitis.12–16 Rituximab has even been reported to be associated with new-onset autoimmune conditions such as vasculitis and psoriasis.17,18 Such an ‘immune stimulatory effect’ has been observed in patients receiving rituximab as part of renal transplant protocol, where the rituximab arm had a much higher incidence of acute cellular rejection than the comparator arm of daclizumab (84 vs. 14%).19 Possible mechanisms before depletion of regulatory B-cells skewing the B-effector and regulatory B-cell balance towards effector cells depending on the timing of B-cell depletion therapy, as has been shown in murine models of multiple sclerosis.20
Paradoxical reactions are well-recognized with biologics such as anti-TNF-alpha, anti-IL17, anti-IL12/23, and anti-IL23 agents. De novo or worsening of pre-existing psoriasis is the prototype paradoxical reaction with TNF-alpha inhibitors, with a variable latency of 15 days to 32 months.21 Other examples of paradoxical reactions include eczematous eruptions, alopecia areata, lupus, sarcoidosis, pyoderma gangrenosum and hidradenitis suppurativa.22
Only a few studies have looked at risk factors for post-rituximab paradoxical pemphigus exacerbation. An earlier retrospective case-control study (n = 68) by Narayanan et al found a shorter disease duration (21 vs. 50 months, p = 0.03), higher baseline body surface area (10 vs. 3%, p < 0.001), more frequent secondary bacterial infection (67 vs. 10%, p < 0.001) and less frequent mucosal involvement (56 vs. 78%, p < 0.001) to be associated with exacerbation after rituximab.7 Bhattacharjee et al reported a higher disease severity (mean ABSIS score 26 vs. 12, p = 0.04) in patients who exacerbated.8 We also found pemphigus clinical severity as a predictor of post-rituximab flare. Affected body surface area as well as PDAI scores were higher in the group that exacerbated, but the difference was statistically significant for PDAI only. PDAI is a better marker of pemphigus severity than affected body surface area.23 Among the immunological factors, baseline high serum anti-Dsg1 levels, but not anti-Dsg3, anti-AchRM3 or peripheral CD19+ B cell population, were found to be associated with post-rituximab flare. While the relationship between serum anti-Dsg levels and pemphigus severity is well-established, anti-AchR antibodies have recently gained attention.24,25 However, we did not find a statistically significant association of anti-AchRM3 levels with disease severity.
The combination of PDAI score (>28) and anti-Dsg1 levels (>1137 RU/ml) was found to have a positive predictive value of 50% and a good negative predictive value of 87.5% for post-rituximab paradoxical flare. In the absence of serum anti-Dsg1 levels before PDAI score (positive predictive value 38%, negative predictive value 93%) alone may be used to guide clinical decision-making.
The strengths of our study include its prospective cohort design, use of validated measures of pemphigus severity, and evaluation of immunological parameters. In the absence of a validated definition, we used a clinically meaningful criterion for post-rituximab flare in this study.
Limitations
Being from a single centre, our study has a relatively small sample size. Though it appears likely that these unexpected pemphigus flares are associated with rituximab infusion, the possibility of spontaneous disease exacerbation cannot be entirely excluded. Finally, immunological factors were not evaluated at the time of the pemphigus flare, which could have shed some insight into the pathophysiology of the post-rituximab paradoxical flare.
Conclusion
Paradoxical pemphigus flares after rituximab occurs in about a fifth of the patients. Patients with more severe pemphigus or high serum anti-Dsg1 levels are at risk of such a post-rituximab flare and may benefit from rituximab administration under close monitoring.
Ethical approval
This was a prospective observational cohort study conducted at the All India Institute of Medical Sciences (AIIMS), New Delhi, India, from August 2021 to October 2022. The protocol was registered (CTRI/2020/10/028675) and approved by the Institute Ethics Committee.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
This study was financially supported by the Intramural Research Grant at the All India Institute of Medical Sciences, New Delhi, India (Project code A-828).
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
References
- Updated S2K guidelines on the management of pemphigus vulgaris and foliaceus initiated by the European academy of Dermatology and Venereology (EADV) J Eur Acad Dermatol Venereol. 2020;34:1900-13.
- [CrossRef] [PubMed] [Google Scholar]
- First-line rituximab combined with short-term prednisone versus prednisone alone for the treatment of pemphigus (Ritux 3): A prospective, multicentre, parallel-group, open-label randomised trial. Lancet. 2017;389:2031-40.
- [CrossRef] [PubMed] [Google Scholar]
- Successful treatment of pemphigus with biweekly 1-g infusions of rituximab: A retrospective study of 47 patients. J Am Acad Dermatol. 2013;68:404-11.
- [CrossRef] [PubMed] [Google Scholar]
- Paradoxical worsening of pemphigus vulgaris following rituximab therapy. Br J Dermatol. 2015;173:858-9.
- [CrossRef] [PubMed] [Google Scholar]
- Paradoxical reaction to rituximab in patients with pemphigus: A report of 10 cases. Immunopharmacol Immunotoxicol. 2020;42:56-8.
- [CrossRef] [PubMed] [Google Scholar]
- Drug-induced pemphigus: A systematic review of 170 patients. Int Immunopharmacol. 2021;92:107299.
- [CrossRef] [PubMed] [Google Scholar]
- A retrospective case-control study of clinical factors associated with paradoxical exacerbation of pemphigus vulgaris following rituximab infusion. Int J Dermatol. 2020;59:e459-e460.
- [CrossRef] [PubMed] [Google Scholar]
- Assessment of the effects of rituximab monotherapy on different subsets of circulating T-regulatory cells and clinical disease severity in severe pemphigus vulgaris. Dermatology. 2016;232:572-7.
- [CrossRef] [PubMed] [Google Scholar]
- Assessing the correlation between disease severity indices and quality of life measurement tools in pemphigus. Front Immunol. 2019;10:2571.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- International pemphigus study group. Calculation of cut-off values based on the autoimmune bullous skin disorder intensity score (ABSIS) and pemphigus disease area index (PDAI) pemphigus scoring systems for defining moderate, significant and extensive types of pemphigus . Br J Dermatol. 2016;175:142-9 .
- [CrossRef] [PubMed] [Google Scholar]
- Pemphigus Oral Lesions Intensity Score (POLIS): A novel scoring system for assessment of severity of oral lesions in pemphigus vulgaris. Front Med (Lausanne). 2020;7:449.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Dramatic exacerbation of bullous pemphigoid following rituximab and successful treatment with omalizumab. Eur J Dermatol. 2019;29:213-5.
- [CrossRef] [PubMed] [Google Scholar]
- Disease exacerbation after rituximab induction in neuromyelitis optica. Neurol Neuroimmunol Neuroinflamm. 2015;2:e61.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Ocular and orbital exacerbation after rituximab therapy for granulomatosis with polyangiitis. Can J Ophthalmol. 2019;54:e237-e241.
- [CrossRef] [PubMed] [Google Scholar]
- Immunoglobulin M ‘Flare’ seen in a case of waldenstrom’s macroglobulinemia: successfully managed by therapeutic plasma exchange. Indian J Hematol Blood Transfus. 2016;32:148-51.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Rituximab-associated flare of cryoglobulinemic vasculitis. Kidney Int Rep. 2021;6:2840-49.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Rituximab-induced vasculitis: A case report and review of the medical published work. J Dermatol. 2009;36:284-7.
- [CrossRef] [PubMed] [Google Scholar]
- Rituximab-induced psoriasis in a patient with granulomatosis with polyangitis treated with adalimumab. Case Rep Rheumatol. 2019;5450863
- [Google Scholar]
- B-cell-depleting induction therapy and acute cellular rejection. N Engl J Med. 2009;360:2683-5.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Regulatory B cells inhibit EAE initiation in mice while other B cells promote disease progression. J Clin Invest. 2008;118:3420-30.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Paradoxical reactions to biologic therapy in psoriasis: A review of the literature. Actas Dermosifiliogr (Engl Ed). 2018;109:791-800.
- [CrossRef] [PubMed] [Google Scholar]
- Paradoxical reactions to biologicals for psoriasis. Expert Opin Biol Ther. 2022;22:1435-7.
- [CrossRef] [PubMed] [Google Scholar]
- Severity score indexes for blistering diseases. Clin Dermatol. 2012;30:108-13.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Correlation of antimuscarinic acetylcholine receptor antibody titers and antidesmoglein antibody titers with the severity of disease in patients with pemphigus. J Am Acad Dermatol. 2017;76:895-902.
- [CrossRef] [PubMed] [Google Scholar]
- Effectiveness of topical pilocarpine in refractory oral lesions of pemphigus vulgaris: Results from an open-label, prospective, pilot study. Dermatol Ther 2022:e15449.
- [CrossRef] [PubMed] [Google Scholar]






