Translate this page into:
Clinical, bacteriological, and histopathological characteristics of newly detected children with leprosy: A population based study in a defined rural and urban area of Maharashtra, Western India
2 Kushthrog Nivaran Samiti, Shantivan, Panvel, Raigad, India
3 Lok Seva Sangam, D1, Everad Nagar Sion-Chunabatti, Mumbai, India
Correspondence Address:
Vanaja P Shetty
The Foundation for Medical Research, 84-A, R G Thadani Marg, Worli, Mumbai - 400 018
India
| How to cite this article: Shetty VP, Ghate SD, Wakade AV, Thakar UH, Thakur DV, D'souza E. Clinical, bacteriological, and histopathological characteristics of newly detected children with leprosy: A population based study in a defined rural and urban area of Maharashtra, Western India. Indian J Dermatol Venereol Leprol 2013;79:512-517 |
Abstract
Background: Leprosy has been a major public-health problem in many developing countries for centuries. According to the National Leprosy Elimination Programme report of March 2012, there were a total of about 0.13 million cases of leprosy in India, 9.7% of which were children. Numerous studies have investigated child leprosy amongst reported cases however, studies pertaining to proportion and characteristics of undetected childhood cases in the community are very few. Aim: To examine the clinical, bacteriological, and histopathological characteristics of newly detected child leprosy cases in the community. Methods: The population survey conducted from June to September 2007 and the defined rural areas, which included five primary health centers of Panvel Taluka, in Raigad district and urban areas, which included M-east ward of the municipal corporation of greater Mumbai of western Maharashtra, India. Results: House-to-house survey yielded 32 and 37 so far, undetected child cases of leprosy in the rural and urban region, and the prevalence rate was 10.5 and 1.5 per 10,000, respectively. The age of child leprosy cases detected, ranged from 3 to 14 years with a mean of 10.06 ± 3.35 years in the rural and 9.97 ± 3.12 years in the urban area. Most of the cases were paucibacillary (62%). A large proportion of children (49%) had single skin lesion (SSL). Of the 19 SSL cases examined histopathologically, 15 (99%) showed features of borderline tuberculoid, 1 (5%) borderline lepromatous and 3 (16%) had indeterminate type of leprosy. Tuberculoid leprosy was not seen in any, indicating less likelihood of self-healing. Overall, three cases had deformity (grade 1 = 1 and grade 2 = 2) and 31% of multibacillary cases were smear positive. Conclusion: The clinical, bacteriological, and histopathological characteristics of newly detected child cases in the community evidently indicate the grave nature of the problem of undetected child leprosy, recent active transmission, and highlight implications on individual patients and the community. Key Message: Most of the cases were paucibacillary (62%). A large proportion of children (49%) had SSL and (55%) had it on the face followed by arms and leg (27%) and trunk (17%). The mean duration of symptoms exceeded one year which can be attributed to poor knowledge of leprosy or barriers in access to health care or its utilization.Introduction
Leprosy has been a major public-health problem in many developing countries for centuries. Children are believed to be the most vulnerable group to Mycobacterium leprae infection and clinical manifestation is often seen in adolescence or young adulthood following the long incubation period. [1],[2] The crucial role of frequency of leprosy in children as an indicator of the level of transmission in the community has been acknowledged. [3]
The World Health Organization (WHO) found a wide variation in the proportion of children amongst newly detected cases in different regions. In 2007, in Africa, this proportion ranged from 2.89% in Togo to 37.96% in the Comoros. Within America, 14.02% of the Dominican Republic′s and 0.32% of Argentina′s new leprosy cases were children. South-East Asia, on the other hand showed a narrower range with Nepal reporting 3.34% compared to 14.1% in Timor-Leste. [4]
Fine [5] scrutinized the Global leprosy situation of 2006 and contemplated if the variation in case detection in younger age groups reflected the actual trend in infection. He deliberated that the observed trend could be the outcome of difference in case detection methods (primary surveys in schools) or lack of standard age criterion for the child category in different countries. A multistage cluster sampling survey in north Bangladesh demonstrated that the prevalence of previously undiagnosed leprosy in children (5-14 years old) was 8.6 per 10,000. [6] In the pediatric age group, leprosy often presents as a single hypopigmented patch, which may or may not be anesthetic and is hence, confused with other dermatological ailments. [7]
India accounts for 55% of the new leprosy cases detected globally in 2010. [8] According to the National Leprosy Elimination Programme report of March 2012, there were a total of about 0.13 million cases of leprosy in India, 9.7% of which were children. Maharashtra state contributed for about one fifth of the leprosy cases and within Maharashtra, reports have consistently shown wide variation in reported leprosy cases in various districts. [9] In a prospective study carried out in a tertiary facility in Southern-Gujarat (March 1999 to March 2002), 8.4% of new leprosy cases were found to be children. [10] Sardana, in his retrospective hospital based study in Northern-India, found 86 children (0-15 years old) amongst the 1115 leprosy cases (7.71%) detected between 1992 and 2003. [7] Though these studies provide information on child leprosy amongst reported cases, the information regarding the clinical, bacterial, and histopathological characteristics of child leprosy cases in the community are very few.
Methods
This study is aimed at estimating the burden of undetected active child leprosy cases in the community in the rural and urban region of western Maharashtra, India and outlining the clinical, bacteriological, and histopathological characteristics of newly detected child cases. This is part of a total population based survey conducted in a defined area and aimed to estimate the prevalence of undetected active cases of leprosy including, children in the community in the rural and urban region of western Maharashtra, India. The rural area included Panvel Taluka, of Raigad district, which had a population of 196,694, in this study. The study area consisted of areas catered by five primary health centers. A primary health center represents the second tier of the three tier rural public health-care system in India and caters to a population of about 20,000-30,000 as per the population norms of the Ministry of Health and Family Welfare. The urban setup included Mumbai city which has 24 administrative units of the municipal corporation of greater Mumbai (A-T) called wards. M-east ward located in the north-eastern part of Mumbai was investigated in this study. The study area with a population of 600,247 was stratified into nine areas based on the health posts catering to them and analysis was conducted accordingly. A health post is the urban counterpart of a primary health center, which caters to a population of about 50,000.
A primary household based survey was conducted from June to September 2007 during which trained health workers screened the adults and children (age <14 years) within their respective areas to detect all active untreated cases of leprosy. Following this, a mop up survey was conducted in October-November 2007 to cover the households that had been missed during the first round of the survey. Provisionally diagnosed cases including children were referred to the medical facility maintained by the investigating institute in the field area for confirmation by clinical, bacteriological, and histopathological examination.
A semi-structured interview schedule was administered to the parents/guardians of the children after obtaining an informed verbal consent. This interview schedule enquired into the socio-demographic details, signs and symptoms experienced by the children and their duration. The children were examined clinically by a dermatologist (Dr SD Ghate), to further ascertain and classified as paucibacillary (PB), that is, <5 lesions and multibacillary (MB), that is, >5 lesions as per WHO working classification. [11] A slit skin smear (SSS) examination was performed using sterile scalpel blades by trained paramedical staff using standard techniques. The air dried and heat fixed smear was stained by Ziehl Neelson carbol fuschin stain and graded as per Ridley′s scale for bacteriological index (BI). [12]
A skin lesional biopsy was obtained using local anesthesia, following verbal consent from the patient (minor), and written informed consent from the guardian. In one case that showed only sensory impairment in the distribution of right sural nerve, right sural nerve biopsy was obtained. This study was approved by the Institutional Ethics Committee.
Results
Survey findings and prevalence rate (PR) of child leprosy in rural and urban areas
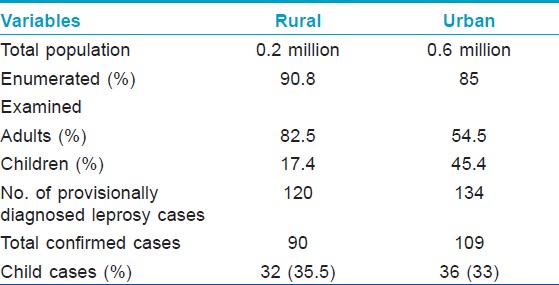
Total population enumerated/examined, during the survey and the proportion of children among them in rural and urban area is depicted in [Table - 1]. A total of the 199 new cases were detected during survey of which 68 (34.1%) were children. The overall prevalence rate (PR) was 5 and 2 per 10,000 and childhood cases PR was 10.5 and 1.54 per 10,000 in rural and urban area respectively. According to the figures published by Government of India, the PR was 1.7 in Raigad district and 0.9 per in urban area. [13]
Rural area
Of the 35 rural provisionally diagnosed childhood leprosy cases, 32 were confirmed as having leprosy on clinical examination of which 21 were male and 11 were females. The age of the child hood cases detected during this study ranged from three to 14 years. The mean age was 10.06 ± 3.35 years. The mean duration of symptoms was reported to be about 14.5 months (range 1-96 months).
Clinical findings
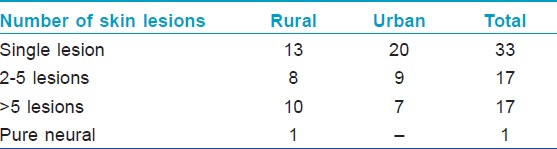
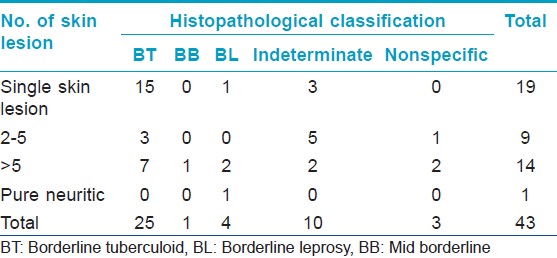
A majority of the child hood cases in the rural population were PB (21/32). Among the PB cases, 28% (13/32) had single skin lesion (SSL) of which 54% (7/13) had lesion on the face [Figure - 1] and [Figure - 2] [Table - 2]. One pure neural case was detected and sural nerve biopsy was performed. On examination for the grade of deformity, it was found that 29 children had no deformity, one patient had grade 1 and two had grade 2 deformity. Thus, about 9% of child leprosy cases in the rural study population had deformity grade 1 or 2. None presented with type 1 or 2 lepra reactions.
 |
| Figure 1: A 10-year-old child with single hypopigmented lesion (?#195;) and few satellite lesions. Mother is a relapse case with histoid lesion (→ ) |
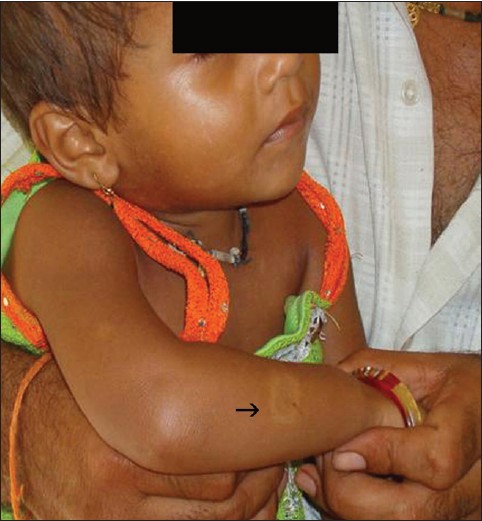 |
| Figure 2: A 3-year-old child with a hypopigmented patch (→ ) over right arm. Father was a case of borderline tuberculoid leprosy |
Bacteriological and histopathological findings
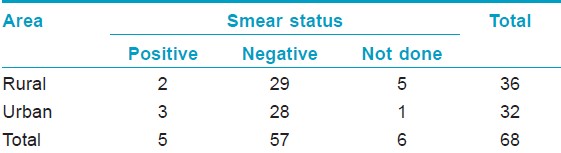
On examination for bacteriological status using SSS, it was found that, 2/10 (20%) MB cases were smear positive with a BI of > 2+ [Table - 3]. All the PB cases were smear negative. Of the 21 children examined histopathologically, majority showed characteristic features of borderline tuberculoid (BT) leprosy (11/21), four were borderline leprosy (BL), four indeterminate, and in one case lesion showed nonspecific type of infiltrate [Table - 4]. Of the four (4/7) facial lesions, which were examined histopathologically, three showed features of BT leprosy and one was indeterminate. Notably, tuberculoid (TT) features were not seen in any [Table - 5].
Urban area
Of the 38 urban provisionally diagnosed childhood leprosy cases, 37 were confirmed as leprosy on clinical examination, of which 18 were males and 18 were females. Only one patient out of the 38 was not leprosy clinically. One child was a treatment dropout and was thus, excluded. The mean age was 9.97 ± 3.12 years (range 3-14 years). The mean duration of symptoms was 14.06 months (range 1-60 months).
Clinical findings
Majority of them were PB (29/36). Among the PB cases, 69% (20/29) had SSL [Table - 2] of which 55% (11/20) had lesion on the face There were no children in the urban area with deformity grade 1 or 2. None presented with type 1 or 2 lepra reactions.
Bacteriological and histopathological findings
On examination for bacteriological status using SSS it was found that, 3/6 (50%) of MB cases were smear positive with a BI of 3+ [Table - 3]. All PB cases were smear negative. Of the 22 children who were examined histopathologically, it was found that 14 children had BT, six had indeterminate type of leprosy and two had nonspecific infiltrate not conferring to leprosy [Table - 4]. Of the three (3/11) facial lesions which were examined histopathologically, two showed features of BT leprosy and one was BL [Table - 5].
History of contact in rural and urban area
In the rural study area, 15 (47%) children had history of contact with leprosy patients. Four of them had history of contact with multiple leprosy patients. It was observed that most of the child cases detected from Apta (5/8) and Ajiwali (4/4) areas had history of contact with leprosy patients within the family.
In the urban population, 7 (19%) children had history of contact with leprosy patients, of which two had history of contact with multiple leprosy patients.
Discussion
Numerous studies have been conducted on children with leprosy but a majority of these studies used schools or health facilities such as clinics, tertiary hospitals as their settings or are retrospective studies. [4],[7],[10],[14],[15],[16] In contrast to that, the present study concentrates on community based case detection and it was found that there is a high burden of leprosy in children in both the study areas. This has direct implication on the transmission of the disease. The high male to female ratio amongst child leprosy cases observed in this study is similar to the findings of many studies conducted over the past few decades. [17],[18] The mean duration of symptoms in the rural and urban childhood cases are comparable and exceeded 1 year, which can be attributed to poor knowledge of leprosy or barriers in access to health care or its utilization.
In concurrence with other studies, this study also found a large proportion of children (49%) had SSL and (55%) had it on the face followed by hands and legs (27%) and trunk (17%). Single hypopigmented patch on the face in children has high-risk of misdiagnosis, since there are numerous common causes of hypopigmented patches in children. [7] Notably, all the SSL lesion biopsies were proved to be cases of leprosy, as assessed by histopathology and none of them conferred to TT leprosy indicating progressive nature of the disease and less likelihood of self-resolution. Moreover, one of the facial lesions of SSL and the sural nerve biopsy showed BL leprosy by histopathology, and were clinically classified as PB. Similar findings have been documented in an earlier study wherein 62.5% (30/48) and 12.5% (6/48) of SSL showed cellular characteristic features of BT and [mid - borderline (BB)] BB-BL, respectively in newly detected cases. [19] Another finding was, majority of cases had BT (58.1%) leprosy, which is similar to findings by Kumar et al. (58.3%), Jain et al. (66.3%), and Rao (68%). [20],[14],[21]
It has been documented that there is a four-fold risk of developing leprosy in presence of a contact in the neighborhood and this risk increases to nine fold if there is a contact with leprosy patient within the household. [22] This study found that a large proportion of children with leprosy in rural areas have history of contact with leprosy cases. This is indicative of the high-risk of transmission of leprosy in the rural population.
Not surprisingly, a large proportion of PB (73.5%) cases were observed amongst children in this study. This concurs with the findings in earlier studies. [16],[23] Studies have shown that PB cases are infectious and can contribute to transmission of the disease in the community. [24] In a study using mouse foot pad technique, viable M. leprae were isolated in 48% (100/209) of PB cases. [25] In view of this, the large proportion of PB cases also becomes a matter of concern.
Only three children in rural area (9%) had deformity (grade 1 = 1 and grade 2 = 2) and none in the urban. These findings point to the fact that most of these childhood leprosy cases were detected early during the course of the disease and this can be attributed to the active case detection method employed. Thirty one percent of MB cases were smear positive which is a cause of concern as they also represent major source of infection.
With only three countries having prevalence higher than elimination level for leprosy (Brazil, Nepal, and Timor-Leste), the WHO has acknowledged the challenges faced by countries who have already attained elimination to maintain the political commitment and services particularly at the peripheral level. [4] Two retrospective studies carried out in Hyderabad and Surat cities of India spanning over the past two decades (1990-1999 and 2001-2006), illustrated a decline in new case detection rate in children, while the overall prevalence of leprosy in the city was above elimination level. [14],[15] In another retrospective study carried out in tertiary care hospital settings between 2000 and 2009, childhood leprosy was detected in 5.1% of cases. [23] In contrast, active survey has shown a high proportion of children (34.1%) among the newly detected cases, highlighting the burden of undetected child hood leprosy in the rural and urban region in western Maharashtra, India. This indicates hidden cases as well as continuing active transmission in this community. The characteristics of these undetected childhood cases evidently indicate the grave nature of the problem of undetected childhood leprosy and underline implications at individual and community level. The study also highlights the importance of SSS and biopsy as an aid in diagnosis and classification. Secondly, the need to conduct special selective leprosy screening surveys is emphasized especially, in highly endemic areas.
Acknowledgments
We thank Swiss Emmaus Leprosy relief for funding this study. Drs. Thomas von Stamm from the Swiss Emmaus Leprosy relief and Dinesh Jain (Regional Medical Co-ordinator), Swiss Emmaus Leprosy relief have been very supportive throughout the study. We thank the field team and health workers from both Kustharog Nivaran Samiti and Lok Seva Sangam for helping us in the survey. We would like to acknowledge Dr. Nerges Mistry, Director, The Foundation for Medical Research for her inputs from the conceptualisation to its completion. Our thanks to Dr. K Ramchandran for his help in the study design. Dr. KV Desikan for confirming the histopathological diagnosis. We would also like to express our gratitude towards patients and their families for agreeing to participate in this study.
| 1. |
Bryceson AG, Pfaltzgraff RE. Leprosy. 3 rd ed. Edinburgh: Churchill Livingstone; 1990.
[Google Scholar]
|
| 2. |
Browne SG. The age of onset of leprosy. Int J Lepr 1965;33:267-72.
[Google Scholar]
|
| 3. |
Bechelli LM, Garbajosa PG, Gyi MM, Dominguez VM, Quagliato R. Site of early skin lesions in children with leprosy. Bull World Health Organ 1973;48:107-11.
[Google Scholar]
|
| 4. |
World Health Organization. Global leprosy situation, beginning of 2008. Wkly Epidemiol Rec 2008;83:293-300.
[Google Scholar]
|
| 5. |
Fine PE. Global leprosy statistics: a cause for pride, or frustration? Lepr Rev 2006;77:295-7.
[Google Scholar]
|
| 6. |
Moet FJ, Schuring RP, Pahan D, Oskam L, Richardus JH. The prevalence of previously undiagnosed leprosy in the general population of northwest Bangladesh. PLoS Negl Trop Dis 2008;2:e198.
[Google Scholar]
|
| 7. |
Mahajan S, Sardana K, Bhushan P, Koranne RV, Mendiratta V. A study of leprosy in children, from a tertiary pediatric hospital in India. Lepr Rev 2006;77:160-2.
[Google Scholar]
|
| 8. |
World Health Organization. Global burden of leprosy at the end of 2010. Wkly Epidemiol Rec 2011;86:389-400.
[Google Scholar]
|
| 9. |
NLEP - Progress Report for the year 2011-12 ending on 31 st March 2012. Central Leprosy Division. Directorate General of Health Services Nirman Bhawan, New Delhi - 110 011, 2012. Available from: http://nlep.nic.in/Revised%20Progress%20report%2031 st %20March%202011-12.pdf. [Last accessed on 2012 Dec 27].
[Google Scholar]
|
| 10. |
Vara N. Profile of new cases of childhood leprosy in a hospital setting. Indian J Lepr 2006;78:231-6.
[Google Scholar]
|
| 11. |
WHO Expert Committee on Leprosy. World Health Organ Tech Rep Ser 1998;874:1-43.
[Google Scholar]
|
| 12. |
Ridley DS. The bacteriological interpretation of skin smears and biopsies in leprosy. Trans R Soc Trop Med Hyg 1955;49:449-52.
[Google Scholar]
|
| 13. |
Government of India. National Leprosy Elimination Programme report. Ministry of Health and Family Welfare, Government of India, New Delhi, India. March 2008
[Google Scholar]
|
| 14. |
Jain S, Reddy RG, Osmani SN, Lockwood DN, Suneetha S. Childhood leprosy in an urban clinic, Hyderabad, India: Clinical presentation and the role of household contacts. Lepr Rev 2002;73:248-53.
[Google Scholar]
|
| 15. |
Chudasama RK, Godara N, Tripathi VS, Patel M. An observation of leprosy situation in Surat district from 2001 to 2006. Indian J Dermatol Venereol Leprol 2007;73:434-5.
[Google Scholar]
|
| 16. |
Horo I, Rao PS, Nanda NK, Abraham S. Childhood leprosy: Profiles from a leprosy referral hospital in West Bengal, India. Indian J Lepr 2010;82:33-7.
[Google Scholar]
|
| 17. |
Selvasekar A, Geetha J, Nisha K, Manimozhi N, Jesudasan K, Rao PS. Childhood leprosy in an endemic area. Lepr Rev 1999;70:21-7.
[Google Scholar]
|
| 18. |
John AS, Rao PS, Kundu R, Raju MS. Leprosy among adolescents in Kolkata, India. Indian J Lepr 2005;77:247-53.
[Google Scholar]
|
| 19. |
Shetty VP, Thakar UH, D'souza E, Ghate SD, Arora S, Doshi RP, et al. Detection of previously undetected leprosy cases in a defined rural and urban area of Maharashtra, Western India. Lepr Rev 2009;80:22-33.
et al. Detection of previously undetected leprosy cases in a defined rural and urban area of Maharashtra, Western India. Lepr Rev 2009;80:22-33.'>[Google Scholar]
|
| 20. |
Kumar B, Rani R, Kaur I. Childhood leprosy in Chandigarh; clinico-histopathological correlation. Int J Lepr Other Mycobact Dis 2000;68:330-1.
[Google Scholar]
|
| 21. |
Rao AG. Study of leprosy in children. Indian J Lepr 2009;81:195-7.
[Google Scholar]
|
| 22. |
van Beers SM, Hatta M, Klatser PR. Patient contact is the major determinant in incident leprosy: Implications for future control. Int J Lepr Other Mycobact Dis 1999;67:119-28.
[Google Scholar]
|
| 23. |
Sachdeva S, Amin SS, Khan Z, Alam S, Sharma PK. Childhood leprosy: a retrospective study. J Public Health Epidemiol 2010;2:267-71.
[Google Scholar]
|
| 24. |
Halder A, Mundle M, Bhadra UK, Saha B. Role of paucibacillary leprosy in the transmission of disease. Indian J Lepr 2001;73:11-5.
[Google Scholar]
|
| 25. |
Wakade AV, Shetty VP. Isolation of Mycobacterium leprae from untreated borderline tuberculoid, mid-borderline and indeterminate cases using the mouse foot pad technique - A study of 209 cases. Lepr Rev 2006;77:366-70.
[Google Scholar]
|
Fulltext Views
4,463
PDF downloads
2,432





