Translate this page into:
Clinical features of primary cicatricial alopecia in Chinese patients
Correspondence Address:
Xingqi Zhang
Department of Dermatology, First Affiliated Hospital of Sun Yat sen University, Guangzhou, Guangdong 510080
China
| How to cite this article: Qi S, Zhao Y, Zhang X, Li S, Cao H, Zhang X. Clinical features of primary cicatricial alopecia in Chinese patients. Indian J Dermatol Venereol Leprol 2014;80:306-312 |
Abstract
Background: There have been few reports on primary cicatricial alopecias (PCR) especially from Asia (PCA). Aims: To study the clinical, pathological and dermoscopic characteristics of PCA among Chinese patients. Methods: A retrospective analysis of the clinical data of 59 patients with PCA was conducted and the dermoscopic, pathological, treatment and prognosis characteristics analyzed. Fisher's Chi-square exact test, Kruskal-Wallis and Spearman rank correlation test were performed. Results: The ratio of neutrophilic to lymphocytic cicatricial alopecias was about 1.3:1 in this group. The most frequent disorder was folliculitis decalvans. Follicular openings were absent on dermoscopy in all cases except alopecia mucinosa. Patulous follicular openings were characterisitc of alopecia mucinosa. After treatment, an increase in short vellus hairs was the earliest feature, while telangiectasia, epidermal scale, follicular hyperkeratosis, pustules and hair diameter diversity gradually decreased or even disappeared. Improvement in the areas of hair loss after treatment was seen more often in discoid lupus erythematosus, folliculitis decalvans and dissecting cellulitis than in patients with classic pseudopelade of Brocq. Nine patients (13.6%) relapsed after cessation of therapy. Female patients needed longer treatment times. Long duration, large areas of hair loss and shorter treatment courses were the major factors in relapses. Conclusions: Dermatoscopy provides a rapid, practical and useful aid for the diagnosis of PCA and also to assess disease activity. Patulous follicular openings are a specific dermoscopic sign of alopecia mucinosa. Lichen planopilaris is less common in China than in the West.INTRODUCTION
Primary cicatricial/scarring alopecias (PCA) are a group of rare disorders with permanent hair loss. These may be divided into:
- A lymphocytic group (e.g. chronic cutaneous lupus erythematosus/discoid lupus erythematosus [DLE], lichen planopilaris [LPP], classic pseudopelade of brocq [PB], alopecia mucinosa [AM])
- Neutrophilic group (e.g. folliculitis decalvans [FD], dissecting cellulitis/folliculitis [DC]) and
- A mixed group and nonspecific group according to the histopathologic features. [1]
These alopecias are difficult to treat, seriously affecting the patient′s quality of life. In this retrospective study, we analyzed the clinical, histological, dermoscopic features, and the treatment and prognosis of primary scarring alopecia in 59 Chinese patients.
METHODS
Patients
Between April 2007 and February 2013, 59 cases of PCA were identified among patients attending our Hair Clinic, accounting for 2.1% of all patients with hair loss. The diagnosis was established using clincal and histopathologic criteria; direct immunofluorescence was performed in some cases.
Methods
The age, sex, age of onset, clinical presentation, duration of disease, concomitant diseases, and response to treatment were recorded. Laboratory tests included routine blood tests, double-stranded deoxyribonucleic acid (dsDNA) and antinuclear antibody (ANA) (ELISA, Aesku Diagnostics, Wendelsheim, Germany). Dermatoscopic examination was performed by a hand-held non-contact polarized dermoscope (Dermlite, DL3 model, 3Gen, San Juan Capistrano, CA, USA). The area of hair loss was calculated using the Olsen method. [2] Pus swabs were taken for culture and antibiotic sensitivity test. The scalp biopsy specimens were obtained from the active border of areas of hair loss after obtaining signed consent. Biopsy samples were divided into two parts and horizontal and vertical sections were stained with haematoxylin and eosin. Direct immunofluorescence (DIF) was performed in 16 cases. Alcian blue staining for mucin was done in 3 cases.
Treatment regimens
The treatment period varied from 3 to 48 months with an average of 10.3 months. Patients with lymphocytic PCA were given oral methylprednisolone tablets 0.5 mg/kg for 4 weeks along with hydroxychloroquine in some patients. The dose was then gradually reduced. For patients with neutrophilic PCA, oral antimicrobial therapy was given based on antimicrobial sensitivity results and isotretinoin was administered to patients with dissecting cellulitis.
Statistical analysis
The collected data were analyzed using SPSS version 16.0. Fisher′s Chi-square exact test and Kruskal-Wallis test were performed for inter-group comparisons. Associations between sex, disease course, onset age, the improvement in hair loss area (the percentage of reduced area in the original hair loss area), treatment course and area of hair loss were analyzed using Spearman rank correlation test. A P value less than 0.05 was considered statistically significant.
Results
Clinical findings
The study comprised of 36 male and 23 female patients (≈1.6:1), aged 5 to 74 years with mean age of 31.7 years. Duration of disease ranged from 1 month to 10 years with an average of 20.1 months. The patients included 13 cases of discoid lupus erythematosus, 2 cases each of lichen planopilaris and alopecia mucinosa, 9 with pseudopelade of brocq, 24 of folliculitis decalvans, and 9 cases of dissecting cellulitis. The ratio of biopsies with lymphocytic versus neutrophilic infitrates was about 0.79 (26 vs. 33). The clinical features of these patients are summarized in [Table - 1], [Figure - 1] and [Figure - 2].
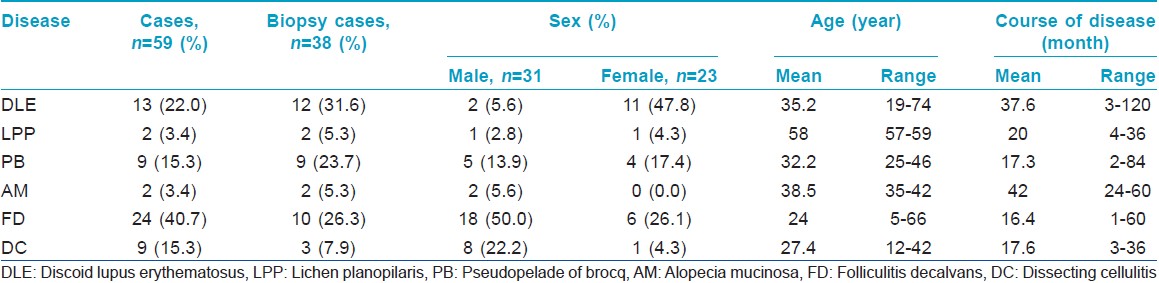
 |
| Figure 1: Global view (a-c), dermoscopy (d-f) and pathological features (g-i) of discoid lupus erythematosus, lichen planopilaris, classic pseudopelade of brocq |
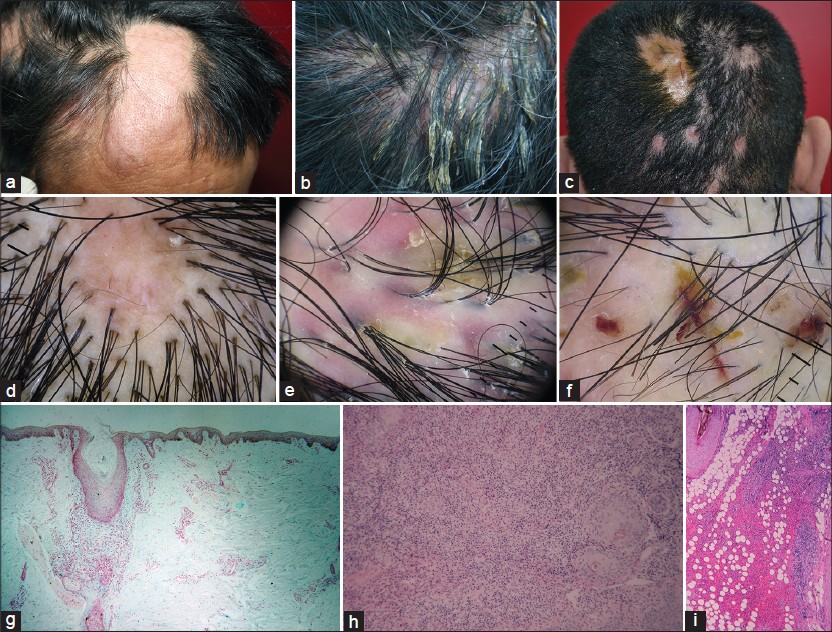 |
| Figure 2: Global view (a~c), dermoscopy (d~f), and pathological features (g~i) of alopecia mucinosa, folliculitis decalvans, dissecting cellulitis/folliculitis |
About 58% of PCA patients had earlier been misdiagnosed as having conditions with non-cicatricial hair loss. The rate of misdiagnosis was highest in patients with lichen planopilaris (100%), followed by pseudopelade of brocq and dissecting cellulitis (78%), and least in folliculitis decalvans (about 45%). The most common misdiagnosis was alopecia areata followed by androgenic alopecia in patients with lymphocytic cicatricial alopecia, whereas the most common misdiagnosis in patients with neutrophilic cicatricial alopecia was folliculitis.
Discoid lupus erythematosus
The hair loss was most often on the parietal scalp (92.3%). Most alopecia patches were round and atrophic, with clear boundary, scales, and hyper- or hypopigmentation. Symptoms were mild. Some patients complained of tickling in the lesion (5 cases, 38.5%), and 3 patients (23%) had occasional knee pain. In addition to scalp lesions, four (30.8%) patients had lesions on other parts of the body including face, trunk, and hands. ANA was positive in 4 (30.8%) cases [Figure - 1]a.
Lichen planopilaris
All involved sites of hair loss were located on the vertex. Erythema with pigmentation, erythema, follicular hyperkeratosis and scattered hair were found in the lesion. No lesions were found in the mouth or on other body sites [Figure - 1]b.
Pseudopelade of Brocq
Areas of alopecia were multifocal, asymptomatic, with depigmentation or hypopigmentation and no obvious erythema or papules. One case also suffered from androgenetic alopecia (AGA) [Figure - 1]c.
Alopecia mucinosa
The plaques on the scalp were multiple, indurated, flesh or hazel in color, with patulous follicular ostia and pruritus. Concomitant AGA was diagnosed in one patient [Figure - 2]a.
Folliculitis decalvans
Most patients (21 cases) had symptoms such as itch, pain, and tenderness. Papules, pustules, nodules, exudation and crusts were seen in these patients. Large numbers of pustules were seen in the more severe cases. Visible tufted hairs were present in 3 cases. Concomitant AGA was present in 4 patients and acne in 6 patients [Figure - 2]b.
Dissecting cellulitis
Patients with dissecting cellulitis had multiple inflammatory papules, abscesses, and cysts; pruritus or pain were the main symptoms. Incision was necessary to drain pus in all patients. Four patients had concomitant acne. [Figure - 2]c.
Bacterial culture results
Bacterial culture yielded positive results in 6 cases (25%) of folliculitis decalvans and 1 case (11.1%) of dissecting cellulitis. Staphylococcus aureus, Staphylococcus epidermidis and other bacteria were isolated. All bacteria tested were sensitive to multiple antibiotics. Microorganisms were not detected in the other cases after several cultures.
Dermoscopic signs
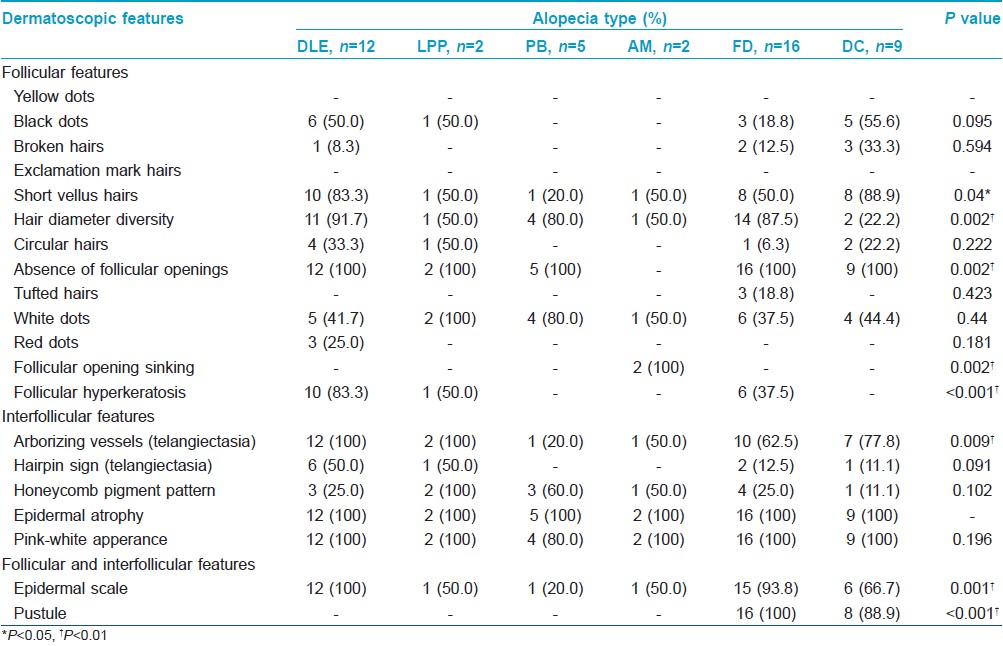
Dermoscopy showed an absence of follicular openings in all patients except the 2 patients with alopecia mucinosa. Epidermal atrophy was seen in all cases and a pink-white appearance noted in most cases [Figure - 1]d-f. Tufted hair was noted only in folliculitis decalvans [Figure - 2]e], and pustules were seen in folliculitis decalvans and dissecting cellulitis [Figure - 2]f]. Patulous follicular openings were only found in alopecia mucinosa [Figure - 2]d. An increase in short vellus hairs was an early feature of response to therapy, while telangiectasia, epidermal scale, follicular hyperkeratosis and pustules and hair diameter diversity gradually decreased or even disappeared during treatment. Significant differences in frequency of dermoscopic signs were noted in different diseases [Table - 2].
Histopathological findings
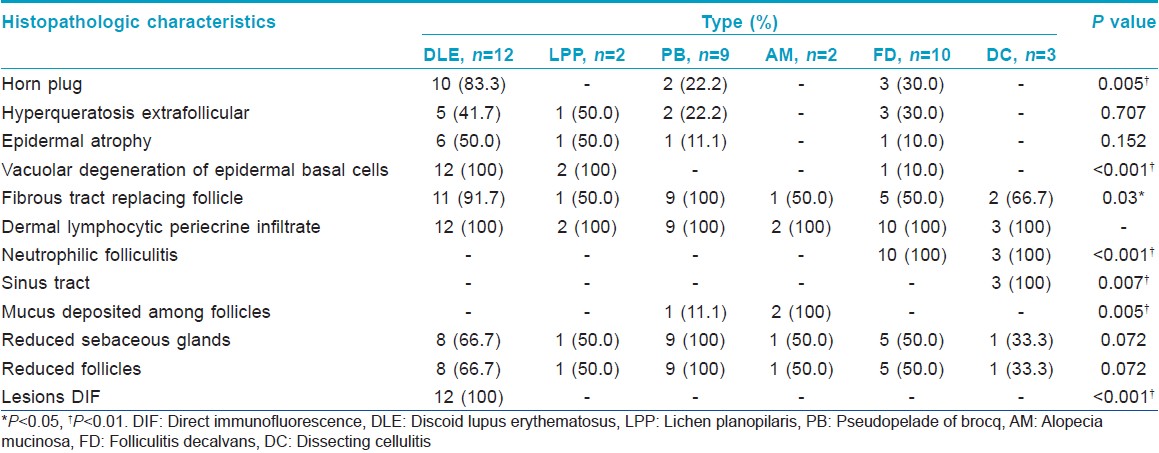
A reduction of the number of sebaceous glands and hair follicles was visible in varying degrees. Hair follicles were replaced by fibrous connective tissue in all patients. The common as well as specific features of the individual diseases are described in [Table - 3]. Vacuolar degeneration of epidermal basal cells, horn plugs and positive DIF were found in discoid lupus erythematosus [Figure - 1]g. Interface dermatitis was characteristic of lichen planopilaris [Figure - 1]h. Minor inflammation was observed in pseudopelade of Brocq, and sebaceous glands were decreased or even absent, with peri-follicular fibroplasia and formation of scar tissue [Figure - 1]i. Hypertrophic arrectores pilorum were noted in two patients with pseudopelade of Brocq. Mucin deposition in the hair follicles was seen in alopecia mucinosa [Figure - 2]g. The formation of neutrophilic abscess was a feature in folliculitis decalvans and dissecting cellulitis [Figure - 1]h and i.
Treatment efficacy and prognosis
Signs of disease control included subsidence of inflammation and a halt in further progression of hair loss. After full courses of treatment, newly grown hair appeared and areas of hair loss were reduced in discoid lupus erythematosus, folliculitis decalvans, and especially in dissecting cellulitis.
Four patients with discoid lupus erythematosus and two cases each of folliculitis decalvans and dissecting cellulitis (13.6% of the treated patients) relapsed after cessation of therapy, but achieved satisfactory results again after repeated treatment. There were significant differences in the degree of improvement with treatment among the various disease entities (P < 0.001). Improvement after the treatment was better in discoid lupus erythematosus, folliculitis decalvans and dissecting cellulitis than in pseudopelade of Brocq (P < 0.05). Approximately 60% of patients with discoid lupus erythematosus and folliculitis decalvans achieved over 20% improvement in the areas of hair loss while among patients with dissecting cellulitis, about 80% patients improved.
Female patients had longer treatment times than males (r = 0.298, P < 0.05). Age was also positively correlated with onset age (r = 0.954, P < 0.001). In addition, disease course positively correlated with treatment time and hair loss area (r = 0.357, r = 0.348, P < 0.01).
DISCUSSION
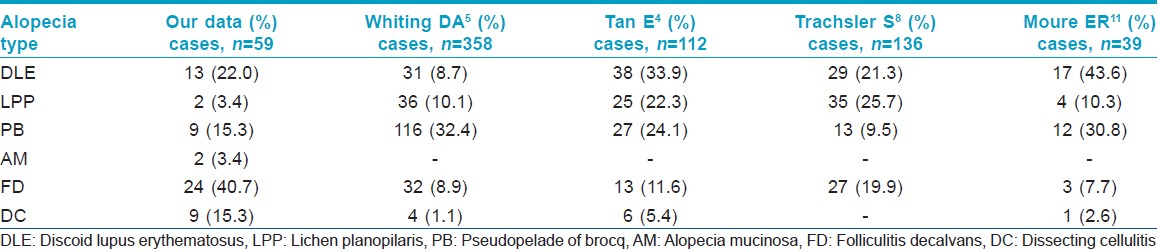
Primary cicatricial alopecia refers to a group of disorders that can result in permanent hair loss due to replacement of follicles by fibrosis or hyalinization of collagen. [3] Primary cicatricial alopecia is folliculocentric with the hair follicle as the main target of an inflammatory process. [4] An earlier study reported that the condition comprised 7.3% of all cases of hair loss, [5] while the frequencey was 3-3.2% in other studies. [4],[6] A recent questionnaire survey in UK revealed about 9.6 new cases were diagnosed per clinician per year. [7] Discoid lupus erythematosus and lichen planopilaris were the most common causes of primary cicatricial alopecia, while folliculitis decalvans and dissecting cellulitis were relatively rare. [6] A histopathologic study of 112 patients found that the majority of cases were lymphocytic, with a ratio of 4:1 to neutrophilic disardess. In this study, discoid lupus erythematosus (33.9%) was the most common, followed by pseudopelade of Brocq (24.1%) and lichen planopilaris (22.3%), and the female to male ratio was 1.6:1. [2] Trachsler and Trueb studied 136 biopsy specimens of scarring alopecia and found that the most frequent diagnosis was lichen planopilaris (26%), followed by discoid lupus erythematosus (21%), folliculitis decalvans (20%), and pseudopelade of Brocq (10%). [8]
Studies on primary cicatricial alopecias from Asia have been sparse [9] and to our knowledge this is the first report from China. In our hair clinic, It constituted 2.1% of patients with hair loss. Folliculitis decalvans (40.7%) was the most frequent diagnosis, followed by discoid lupus erythematosus (22.0%), dissecting cellulitis (22.3%) and pseudopelade of Brocq (22.3%). Lichen planopilaris is one of the most frequent causes of adult primary cicatricial alopecias, [10] but accounted for only 3.4% of patients in our study. The total male to female ratio was 1.6:1 in our study. Middle-aged women were more the affected with lymphocytic cicatricial alopecia whereas middle-aged men suffered more from neutrophilic type. Folliculitis decalvans was more common than dissecting cellulitis in our study, consistent with earlier reports. [4],[5],[7],[11],[12] The proportion of folliculitis decalvans (40.7%) in our study was much higher compared to previous reports (11.6%, 7.7%). [4],[11] Mixed cicatricial alopecia was not observed in our hair clinic. These differences in incidence [Table - 4] may be related to differences in time frame of survey, territorial limitation, or ethnic factors.
Dermatoscopy provides a noninvasive technique to assess scalp and hair characteristics offering important clues for correct diagnosis and biopsy localization. [13],[14] Dermatoscopy of scarring alopecia is characterized by decreased hair density and loss of follicular openings in almost 100% cases. [13],[15] Dermatoscopic appearances in alopecia mucinosa have been described as erythematous, well-defined, indurated plaques with prominent follicular openings and loss of hair. [13],[15] However, we observed retention of follicular openings, which were patulous, in our patients of alopecia mucinosa. Patulous follicular openings were a unique feature of alopecia mucinosa and we propose this as a diagnostic dermatoscopic sign of alopecia mucinosa. The formation of patulous follicular openings may be related to mucin deposition and lymphocytic infiltration in the inter-follicular space and the inelastic follicular tract. Follicular red dots are a specific feature of active discoid lupus erythematosus of the scalp which can help the clinician to differentiate discoid lupus erythematosus from other diseases. [16] This sign was present only in 3 (25%) of 12 patients with a discoid lupus erythematosus with shorter disease course, which is less than previously reported (38%). [16] The characteristic dermoscopic finding of folliculitis decalvans, [17] tufted hairs, was only found in three patients. These three cases all had positive bacterial cultures, longer treatment courses, as well as relapses. The finding of tufted hair may be related to the severity of folliculitis decalvans. [14] In all patients, increase in short vellus hairs was an early feature during treatment, and keratotic plugs, scaling, telangiectasia and pustules decreased or cleared after treatment in active lesions.
Biopsies were less often performed in patients with folliculitis decalvans and dissecting cellulitis where the distinctive clinical and dermatoscopic features were diagnostic and this may have led to an increase in the biopsies with a lymphocytic infiltrate. Scalp biopsies are crucial for the accurate diagnosis and differentiating of cicatricial alopecia and for an estimate of disease activity, especially in lymphocytic disorders. [18],[19] Pathological features of these diseases are mostly consistent with previous reports, [20] apart from the exception that we found hypertrophic arrectores pilorum in a patient with pseudopelade of Brocq, which may be involved in the pathogenesis.
Female patients needed longer treatment as compared to male patients. This may be related to the disease, as well as the psychological stress, which can be more in females than males in Chinese patients. The treatment outcomes of patients with discoid lupus erythematosus, folliculitis decalvans and dissecting cellulitis were better than that of pseudopelade of Brocq, which may be related to marked fibrosis, fewer remaining follicles and lack of inflammatory infiltrate. In this study, 13.6% of patients had relapses after cessation of treatment. Possible factors in such relapses may include longer disease duration, larger areas of hair loss and shorter treatment courses (due to non-compliance). Fear of long-term adverse effects of drugs especially corticosteroids and antibiotics and the financial burden of long treatment courses were among the main reasons for non-compliance or the termination of therapy.
A limitation of this study is the small number of patients in each type of PCA.
Approximately 60% of patients were misdiagnosed as alopecia areata (AA) or other non-scarring alopecia. Primary cicatricial alopecias are uncommon disorders and remain a problem in diagnosis even for dermatologists. Continuing medical education and increasing the awareness is necessary to improve this situation.
| 1. |
Olsen EA, Bergfeld WF, Cotsarelis G, Price VH, Shapiro J, Sinclair R, et al. Summary of North American Hair Research Society (NAHRS)-sponsored workshop on cicatricial alopecia, Duke University Medical Center, February 10 and 11, 2001. J Am Acad Dermatol 2003;48:103-10.
[Google Scholar]
|
| 2. |
Olsen EA, Hordinsky MK, Price VH, Roberts JL, Shapiro J, Canfield D, et al. Alopecia areata investigational assessment guidelines--Part II. National Alopecia Areata Foundation. J Am Acad Dermatol 2004;51:440-7.
[Google Scholar]
|
| 3. |
Somani N, Bergfeld WF. Cicatricial alopecia: Classification and histopathology. Dermatol Ther 2008;21:221-37.
[Google Scholar]
|
| 4. |
Tan E, Martinka M, Ball N, Shapiro J. Primary cicatricial alopecias: Clinicopathology of 112 cases. J Am Acad Dermatol 2004;50:25-32.
[Google Scholar]
|
| 5. |
Whiting DA. Cicatricial alopecia: Clinico-pathological findings and treatment. Clin Dermatol 2001;19:211-25.
[Google Scholar]
|
| 6. |
Abal-Díaz L, Soria X, Casanova-Seuma JM. Scarring alopecia. Actas Dermosifiliogr 2012;103:376-87.
[Google Scholar]
|
| 7. |
Griffin LL, Michaelides C, Griffiths CE, Paus R, Harries MJ. Primary cicatricial alopecias: A U.K. survey. Br J Dermatol 2012;167:694-7.
[Google Scholar]
|
| 8. |
Trachsler S, Trueb RM. Value of direct immunofluorescence for differential diagnosis of cicatricial alopecia. Dermatology 2005;211:98-102.
[Google Scholar]
|
| 9. |
Kumar U M, Yelikar BR.The spectrum of histopathological lesions in scarring alopecia: a prospective study. J Clin Diagn Res 2013;7:1372-6.
[Google Scholar]
|
| 10. |
Assouly P, Reygagne P. Lichen planopilaris: Update on diagnosis and treatment. Semin Cutan Med Surg 2009;28:3-10.
[Google Scholar]
|
| 11. |
Moure ER, Romiti R, Machado MC, Valente NY. Primary cicatricial alopecias: A review of histopathologic findings in 38 patients from a clinical university hospital in Sao Paulo, Brazil. Clinics (Sao Paulo) 2008;63:747-52.
[Google Scholar]
|
| 12. |
Chandrawansa PH, Giam YC. Folliculitis decalvans: A retrospective study in a tertiary referred centre, over five years. Singapore Med J 2003;44:84-7.
[Google Scholar]
|
| 13. |
Dogra S, Sarangal R. What's new in cicatricial alopecia? Indian J Dermatol Venereol Leprol 2013;79:576-90.
[Google Scholar]
|
| 14. |
Sillani C, Bin Z, Ying Z, Zeming C, Jian Y, Xingqi Z. Effective treatment of folliculitis decalvans using selected antimicrobial agents. Int J Trichology 2010;2:20-3.
[Google Scholar]
|
| 15. |
Ross EK, Tan E, Shapiro J. Update on primary cicatricial alopecias. J Am Acad Dermatol 2005;53:1-37.
[Google Scholar]
|
| 16. |
Tosti A, Torres F, Misciali C, Vincenzi C, Starace M, Miteva M, et al. Follicular red dots: A novel dermoscopic pattern observed in scalp discoid lupus erythematosus. Arch Dermatol 2009;145:1406-9.
[Google Scholar]
|
| 17. |
17. Tosti A, Torres F. Dermoscopy in the diagnosis of hair and scalp disorders. Actas Dermosifiliogr 2009;100 Suppl 1 :114-9.
[Google Scholar]
|
| 18. |
Miteva M, Tosti A. Dermoscopy guided scalp biopsy in cicatricial alopecia. J Eur Acad Dermatol Venereol 2013;27:1299-303.
[Google Scholar]
|
| 19. |
Otberg N. Primary cicatricial alopecias. Dermatol Clin 2013;31:155-66.
[Google Scholar]
|
| 20. |
Rudnicka L, Olszewska M, Rakowska A, Slowinska M. Trichoscopy update 2011. J Dermatol Case Rep 2011,5:82-8.
[Google Scholar]
|
Fulltext Views
4,809
PDF downloads
2,700





