Translate this page into:
Clinical, immunological profile and follow up of patients with pemphigus: A study from India
2 Department of Medicine, Christian Medical College and Hospital, Vellore, Tamil Nadu, India
3 Department of Biostatistics, Christian Medical College and Hospital, Vellore, Tamil Nadu, India
Correspondence Address:
Renu George
Department of Dermatology, Venereology and Leprosy, Christian Medical College and Hospital, Vellore - 632 004, Tamil Nadu
India
| How to cite this article: Ramassamy S, Agrawal P, Sathishkumar D, Mathew L, Peter JV, Mani T, George R. Clinical, immunological profile and follow up of patients with pemphigus: A study from India. Indian J Dermatol Venereol Leprol 2018;84:408-413 |
Abstract
Background: Pemphigus has a protracted course and multiple factors influence its prognosis. The objective of this study was to describe the epidemiology and clinical profile of pemphigus patients and to study its influence on treatment end points.
Methods: This was a retrospective chart review done in an Indian tertiary care hospital from December 1991 to December 2013. Patients with less than 3 months' follow up and those who had paraneoplastic pemphigus were excluded.
Results: There were 132 patients with pemphigus, of which 118 (89.4%) had pemphigus vulgaris and 14 (10.6%) had pemphigus foliaceous. The time to disease control (TDC) was available for 100 patients (n = 100, 75.7%); patients with a minimum follow up of 3 months (n = 80) were included for studying the end points like time to first disease remission (TDR) and time to first disease relapse (TDRe). The median period of follow up was 23 months (range 3–245). Out of the 100 patients, 61.9% were on oral steroids with adjuvant therapy. The steroid dose required for disease control for n = 100, ranged from 0.2 to 1.5 mg/kg body weight. Of these, 60% were treated with steroid dose of 1 mg/kg, 22% with >1 mg/kg, and 18% with <1 mg/kg. The mean time to disease control (in months) in the group which received <1 mg/kg steroid was 1.02 ± 0.68, 1 mg/kg was 0.72 ± 0.51, and >1 mg/kg was 1.02 ± 0.62 (P = 0.017); with a significant difference between the groups 2 and 3 (P = 0.007), implying a faster disease control in those who received 1 mg/kg dose. This difference was significant after adjusting for the steroid sparing drugs taken at baseline (P = 0.009, C.I. - 1.44-13.59). The mean time to first disease remission (TDR) was 11.46 ± 2.06 months. Out of the 80 patients with a minimum follow up of 3 months, 75% had achieved either partial or complete remission. None of the other epidemiological, clinical or immunological parameters had an impact on the TDC or TDR.
Conclusions: The epidemiological, clinical or immunological parameters had no impact on the treatment end points like time to disease control and time to first disease remission. The dose of steroids required for disease control higher than 1 mg/kg offered no advantage in the time to disease control as compared to 1 mg/kg.
Limitations: The study was retrospective and disease severity scores were not applied. In view of the shorter follow up period, long term prognostic end points and mortality could not be well represented. The median period of follow up was 23 months. The serum anti- desmoglein antibody titres were not available at various treatment end points for correlation at different time intervals.
Background
Pemphigus has a protracted course marked by remissions and relapses. In India, the prevalence of pemphigus among the dermatology outpatient attendees varies from 0.09 to 1.8%.[1] A number of factors like ethnicity, gender, age at onset, smoking, initial site of disease, initial antibody titers have been implicated in the prognosis of the disease. The study aims to understand the same in our setting by analyzing the follow up data of patients with pemphigus. The objectives of the study were to describe the epidemiological, clinical, and immunological profile of pemphigus patients, to study the influence of these parameters on the various treatment end points and to analyze the follow up data to understand the clinical course of the disease, adverse effects of therapy and mortality.
Methods
This was a retrospective chart review done in the department of dermatology, Christian Medical College, Vellore, South India from December 1991 to December 2013. All patients diagnosed with pemphigus based on the combination of characteristic clinical features along with either of typical histology and/or direct immunofluorescence findings (performed in 119 patients) were included. The exclusion criteria included patients with paraneoplastic pemphigus and childhood pemphigus, as these patients may have different prognostic variables. For the analysis of remission and relapse parameters, patients with less than 3 months follow up were excluded. The follow-up duration was calculated using the number of months elapsed between the patient's first and last recorded visits. The treatment end points applied retrospectively were time to disease control (TDC), time to first disease remission (TDR), and first disease relapse (TDRe) as per consensus statement from the International Pemphigus Committee by Murrell et al.[2] Accordingly, “disease control” was defined as the time at which new lesions ceased to form and established lesions began to heal. The time interval between baseline and control was “Time to disease control (TDC).” Disease remission, either complete or partial was defined as the absence of new or established lesion or presence of transient new lesions that heal within 1 week, respectively, while the patient is on minimal therapy or off therapy. The time interval between baseline and first disease remission was taken as “Time to first disease remission.” A ''relapse'' of disease is defined by the appearance of 3 or more new lesions a month that do not heal spontaneously within 1 week, or by the extension of established lesions, in a patient who has achieved disease control. The time interval between baseline and first disease relapse was “Time to first disease relapse (TDRe).”
Epidemiological factors investigated were age at onset and gender; clinical factors were subtype of disease (pemphigus vulgaris - PV vs. pemphigus foliaceous - PF), site of onset of disease (skin, mucosal or both), initial steroid dose, and steroid dose at relapse. Immunological factors investigated were direct immunofluorescence (DIF), and anti-desmoglein (Dsg) 1 and 3 antibody levels (using Enzyme linked immunosorbent assay (ELISA) kits from MBL Bion Enterprises, Des Plaines, IL USA) at baseline.
For continuous variables we used descriptive statistics (mean and SD). To compare means of TDC with subtype of disease, two independent sample t-test was done. To compare TDC with variables like gender, baseline anti-desmoglein antibody titres (less than or more than 100U), and baseline DIF positivity, Mann–Whitney U test was used. For comparison of TDC across the three steroid dosage groups and site of onset of disease, Kruskal–Wallis test was employed. Logistic regression was used to find the association of time to disease control across the three steroid dose range groups, after adjusting for any adjuvant dose. For correlating TDC with age at onset of disease and for correlating TDC with TDR, Pearson correlation coefficient was used. The study was approved by the Institutional ethics committee (IRB Min No: 9434 (Retro), dated 29-04-2015).
Results
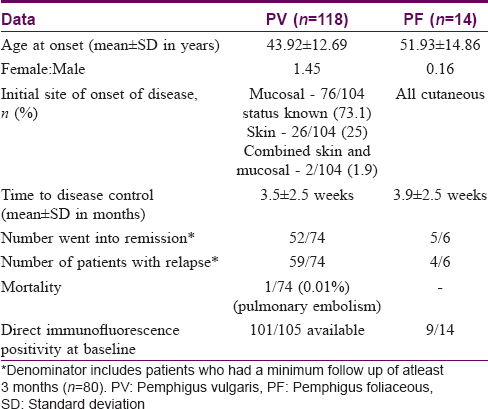
The total number of patients with pemphigus were 132, of which 118 (89.4%) had PV and 14 (10.6%) had PF. The TDC was available for 100 patients; patients with a minimum follow up of 3 months (80 in our study) were included for studying the end points like TDR and TDRe. The profile of patients with PV and PF are listed in [Table - 1].
Prognostic end points
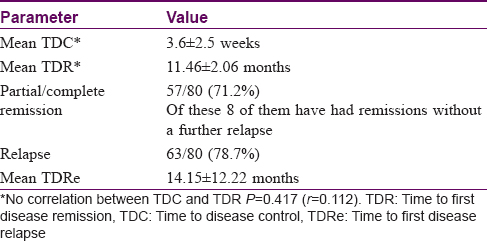
The median period of follow up was 23 months (range 3–242) as depicted in [Figure - 1]. The various prognostic end points observed during the follow-up period are listed in [Table - 2]. The mean TDC was 3.6 ± 2.5 weeks. Fifty-seven patients (57/80, 71.2%) had a remission of disease with the mean TDR being 11.46 ± 2.06 months. The remission period ranged from 3–18 months within the study duration. There was no correlation between TDC and TDR, P = 0.417 (r, 0.112). Sixty-three patients (63/80, 78.7%) had a relapse of disease with mean TDRe being 14.15 ± 12.22 months. One patient (1/80) with pemphigus vulgaris died of pulmonary embolism during the study period.
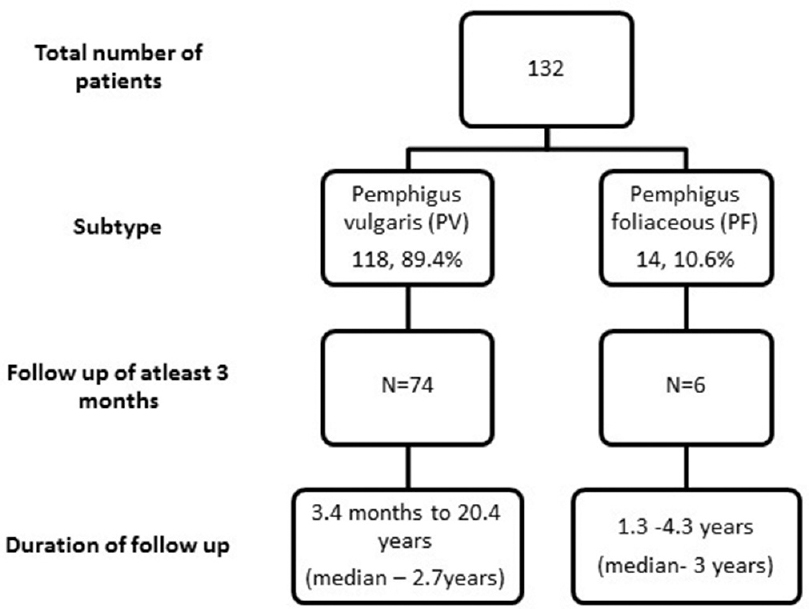 |
| Figure 1: Patient flow chart |
Epidemiological factors versus prognostic end points
The mean age at onset of disease for pemphigus (n = 132) was 44.76 years; for PV was 43.92 ± 12.69 years and that for PF was 51.93 ± 14.86 years and the difference was statistically significant (P = 0.030, C.I.=15.24 to -.78), implying a lower age at onset of disease for those with PV. The female to male ratio was 1.2:1. We did not find any association between age at onset (P = 0.667) or gender (P = 0.367) and TDC.
Clinical factors versus prognostic end points
The commonest subtype of pemphigus was PV (118, 89.4%) and the number of PF patients was 14 (10.6%). The clinical subtype did not have an impact on either TDC (P = 0.550) or TDR (P = 0.888) or TDRe (P = 0.652). The most common site of presentation in PV was mucosal (76/104 status known, 73.1%), followed by cutaneous onset (26/104, 25%) and the least common presentation was a combined skin and mucosal onset (2/104, 1.9%). We were unable to identify an effect of initial site of presentation on TDC (P = 0.134).
Treatment factors versus prognostic end points
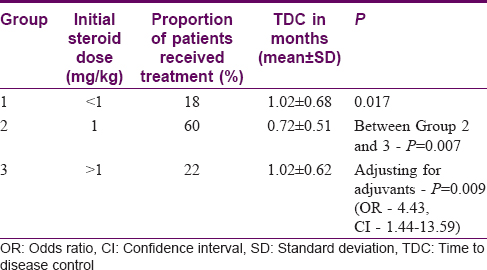
Out of the 100 patients, 61.9% were given oral steroids with immunosuppressants like azathioprine, cyclophosphamide, mycophenolate mofetil and adjuvant therapy like dapsone and/or pyridostigmine. The steroid dose required for disease control ranged from 0.2 to 1.5 mg/kg body weight with 80% of the patients having been treated with 1 mg/kg, 22% with >1 mg/kg, and 18% with <1 mg/kg. The mean TDC in the group which received <1 mg/kg steroid (group 1) was 1.02 ± 0.68 months, 1 mg/kg (group 2) was 0.72 ± 0.51 months, and >1 mg/kg (group 3) was 1.02 ± 0.62 months (across the three groups P = 0.017). Therefore, mean TDC for the group which received <1 mg/kg is same as those who received >1 mg/kg, while those on 1 mg/kg achieved a faster control (statistical difference between the groups 2 and 3, P = 0.007) [Table - 3]. Adjusting for the steroid sparing adjuvants at baseline, patients who were initiated on 1 mg/kg steroid achieved disease control 4 times earlier compared to the other groups (P = 0.009, OR - 4.43, C.I. - 1.44–13.59). However, the initial dose of steroid had no impact on TDR (P = 0.382) or TDRe (P = 0.897). The median dose of steroid at which the first relapse occurred was 5 mg/day (range from 0–52.5 mg).
Immunological factors versus prognostic end points
Direct immunofluorescence of perilesional skin at presentation was positive in 92.4% (110/119 available). No significant association was observed between baseline DIF positivity and TDC (P = 0.917), TDR (P = 0.651), or TDRe (P = 0.481). The baseline anti- desmoglein (Dsg) 1 and 3 antibody levels were categorized as low, if found to be between 20–100U and high if >100U.[3] Of the 84 cases of PV where initial anti-Dsg antibody titres were available, anti-Dsg 1 and 3 antibody titres were elevated in 54.7% (46/84) and 83.3% (70/84) cases respectively. Of the 13 cases of PF for whom anti-Dsg antibody titres were available, all had high anti-Dsg 1 antibody titres and 23% (3/13) had high anti-Dsg 3 antibody. [Figure - 2] and [Figure - 3] depict the proportion of pemphigus patients according to initial anti-Dsg titers. No significant association was observed between baseline anti-Dsg1 antibodies and TDC (P = 0.161), TDR (P = 0.289), or TDRe (P = 0.754) or between anti Dsg3 antibodies and TDC (P = 0.503), TDR (P = 0.333), or TDRe (P = 0.332) in PV. However, the serum anti-desmoglein antibody titres were not available at TDC or TDR for correlation at different time intervals. The impact of various factors on treatment end points has been summarised in [Table - 4].
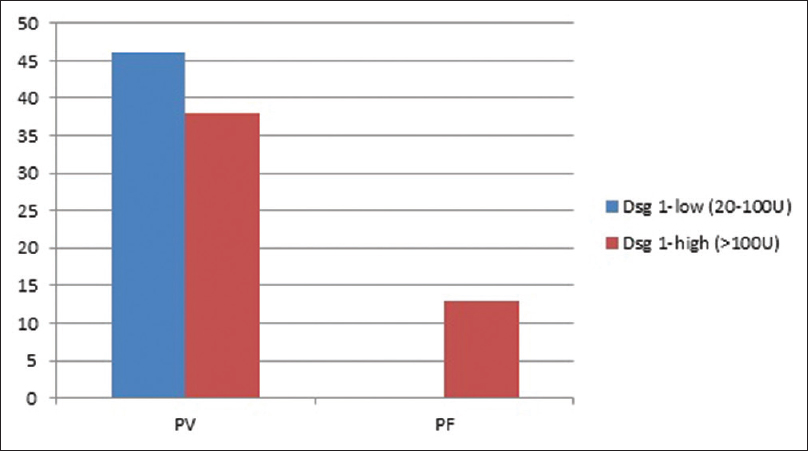 |
| Figure 2: Proportion of pemphigus patients according to initial anti-desmoglein 1 antibody titers |
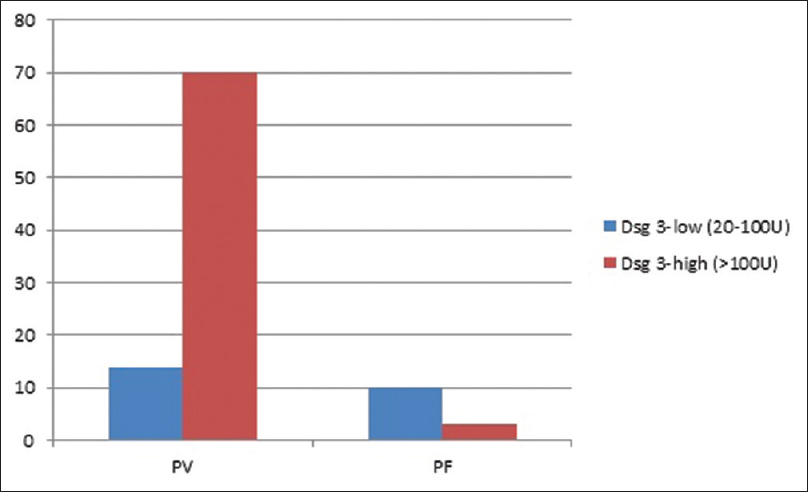 |
| Figure 3: Proportion of pemphigus patients according to initial anti-desmoglein 3 antibody titers |
Adverse effects of treatment
The most common adverse effect of therapy was diabetes mellitus seen in 34.8% (46/132). The other side effects are depicted in [Figure - 4].
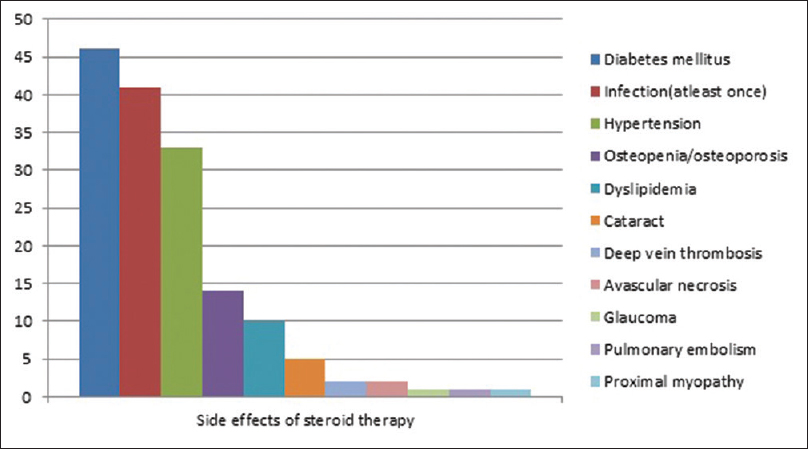 |
| Figure 4: Adverse effects of steroid therapy |
Discussion
The demographic and clinical profile of our patients was comparable to that of other similar studies.[3],[4] In keeping with the original description of the disease, our study also showed that pemphigus affects primarily middle-aged people. The mean age at onset of pemphigus in our study patients was 44.76 years. This was comparable to the age of onset reported in 1209 pemphigus patients from Iran (42 years).[5] The age at onset was significantly lower for patients with PV than PF, which was similar to the observation of Baican et al.,[4] wherein the median age of onset was 50 years in the PV group and 53 in the PF group. A female preponderance was evident in the PV group as reported in other studies.[5] The female to male ratio of patients with pemphigus was 1.2:1 in our study, which was lower than that reported from Iran (1.5:1), but comparable to pemphigus patients from France (1.2:1).[6]
In the past, many studies on pemphigus have identified a number of factors like ethnicity, gender, age at onset, smoking, initial site of disease, initial anti-desmoglein antibody titres in predicting the outcomes in patients with pemphigus setting variable clinical end points like mortality, remission, disease duration, and relapse. Baican et al.,[4] had reported that a late age at onset had a negative impact on the survival in the subjects, which was not evident in our study. A Tunisian study on 47 patients with pemphigus demonstrated that only mucosal involvement at presentation was associated with a shorter median first relapse-free survival time (1.28 years vs. 3 years).[7] Studies also suggest that combined mucocutaneous presentation was associated with a poorer prognosis.[6] In a retrospective study from a single centre in UK done by Saha et al.,[3] there was no association observed between the gender, clinical subtype of disease and initial site of presentation of disease on the end points.[3] However, they highlighted the fact that a higher initial anti-desmoglein 3 antibody level was a predictor of longer disease duration in PV; this was not observed in our series. The comparison between our study and various other studies on prognostic factors in pemphigus is presented in [Table - 5].
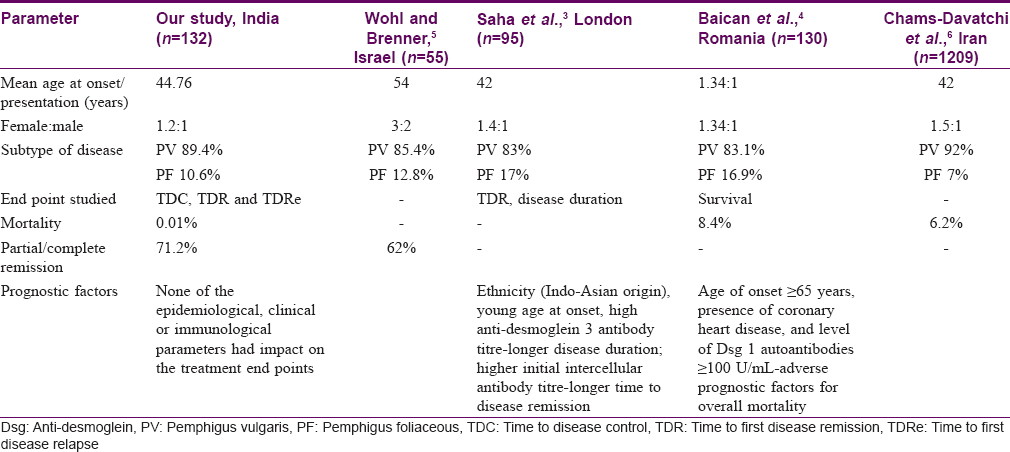
Our study aimed to analyze early end points like TDC and late end points like TDR and TDRe in an Indian setting. There was no association between the age at onset of disease, gender, clinical subtype of disease, initial site of presentation of disease, baseline anti-desmoglein antibody titres or baseline direct immunofluorescence positivity on the therapeutic end points. Our study mostly included middle aged patients, so including children with pemphigus or more elderly patients might have helped us to understand the disease onset in terms of prognostic outcomes better. The titres of baseline circulating intercellular antibodies did not correlate with the disease end points in our study. The possible issues could be in the innate problems with use of ELISA which measures IgG subclass to desmoglein antigens as postulated by Muro et al.[8] Future studies could utilize conformational ELISA or ELISA using subtype-specific antibodies.
Systemic corticosteroids have been the mainstay of clinical management of pemphigus for several decades. However, within the last few years there has been an emergence of treatment modalities that have specific molecular targets. While systemic corticosteroids still remain the mainstay of treatment for PV atleast in resource limited settings, there is no consistency regarding recommended doses.[9] In recent years, the treatment of PV has been shifting away from the long held protocol of treating all patients with high-dose corticosteroids. One randomized controlled trial reported that high-dose oral prednisolone (120–150 mg/d) is not significantly superior to low-dose oral prednisolone (45-60 mg/d) in decreasing relapse rates and increasing duration of remission.[10] The average length of time from initiating therapy to 75% clearance of lesions was 4.5 months in a study by Strowd et al., where the most common starting dose of prednisolone was 1 mg/kg with mycophenolate mofetil.[11] In our study, disease control was achieved in less than a month's time in responders, with mean TDC being 0.84 ± 0.58 months. The initial steroid dose ranged from 0.2 to 1.5 mg/kg with a majority (60%) of patients having received 1 mg/kg. The patients with initial steroid dose of 1 mg/kg achieved faster disease control against those receiving >1 mg/kg (P = 0.009) or <1 mg/kg adjusting for the steroid-sparing agents, suggesting that there is no advantage in a higher dose, similar to the observation made in earlier studies.[10],[12] However there was no significant effect of the initial steroid dose on long term end points. This may be due to the fact that this cohort was derived from a tertiary referral centre and had a high proportion of patients with extensive and recalcitrant disease. Also, disease duration before steroid initiation needed to be analyzed. Further studies must factor in initial disease severity scores, extent of disease, and interval between disease onset and initiation of therapy.
Clinical relapses were common in the first year after therapy discontinuation.[13] Among the 80 patients in our study, the relapse rate was 78% and this occurred at 14 months on average.
The prolonged use of high doses of corticosteroids and immunosuppressants caused several complications, in particular, diabetes, infections, hypertension as observed in other studies.[8] Studies report causes of mortality like sepsis, cardiorespiratory failure in hospitalised patients.[14] The death observed in our study was due to pulmonary embolism and amounts to 0.01% over an average of 2.7 years follow up; however, the mortality reported in studies with longer follow up was 9%.[15]
The limitations of the study were the retrospective nature and that the disease severity scores were not applied. In view of the shorter follow up period, long term prognostic end points and mortality could not be well represented. The serum anti-desmoglein antibody titres were not available at various treatment end points for correlation with disease activity.
Conclusions
The most common subtype was pemphigus vulgaris with a lower mean age of onset of disease as compared to pemphigus foliaceous. Disease control was achieved in almost a month's time and first disease remission was attained in a year's time. None of the epidemiological or clinical factors like age at onset, gender, disease subtype, initial site of presentation, baseline anti-desmoglein antibody titres or baseline direct immunofluorescence positivity had an impact on the treatment end points viz. time to disease control, time to first disease remission and first relapse. Initial steroid dose higher than 1 mg/kg offered no advantage in the time to disease control as compared to 1 mg/kg. Prospective studies that include disease severity scores and serial serum anti-desmoglein antibody titres will add strength to the existing literature.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Kanwar AJ, Vinay K. Treatment of pemphigus: An Indian perspective. Indian J Dermatol Venereol Leprol 2014;80:285-8.
[Google Scholar]
|
| 2. |
Murrell DF, Dick S, Ahmed AR, Amagai M, Barnadas MA, Borradori L, et al. Consensus statement on definitions of disease, end points, and therapeutic response for pemphigus. J Am Acad Dermatol 2008;58:1043-6.
[Google Scholar]
|
| 3. |
Saha M, Bhogal B, Black MM, Cooper D, Vaughan RW, Groves RW, et al. Prognostic factors in pemphigus vulgaris and pemphigus foliaceus. Br J Dermatol 2014;170:116-22.
[Google Scholar]
|
| 4. |
Baican A, Chiorean R, Leucuta DC, Baican C, Danescu S, Ciuce D, et al. Prediction of survival for patients with pemphigus vulgaris and pemphigus foliaceus: A retrospective cohort study. Orphanet J Rare Dis 2015;10:48.
[Google Scholar]
|
| 5. |
Wohl Y, Brenner S. Pemphigus in Israel – An epidemiologic analysis of cases in search of risk factors. Isr Med Assoc J 2003;5:410-2.
[Google Scholar]
|
| 6. |
Chams-Davatchi C, Valikhani M, Daneshpazhooh M, Esmaili N, Balighi K, Hallaji Z, et al. Pemphigus: Analysis of 1209 cases. Int J Dermatol 2005;44:470-6.
[Google Scholar]
|
| 7. |
Khaled A, Taazayet SB, Ben Alaya N, Souissi A, Zeglaoui F, Kaffel N, et al. The course and prognosis of pemphigus in 47 Tunisian patients. J Eur Acad Dermatol Venereol 2013;27:81-5.
[Google Scholar]
|
| 8. |
Muro Y, Sugiura K, Akiyama M. What are the “true” pathogenic anti-desmoglein antibodies? Acta Derm Venereol 2015;95:872, 874.
[Google Scholar]
|
| 9. |
Harman KE, Albert S, Black MM, British Association of Dermatologists. Guidelines for the management of pemphigus vulgaris. Br J Dermatol 2003;149:926-37.
[Google Scholar]
|
| 10. |
Ratnam KV, Phay KL, Tan CK. Pemphigus therapy with oral prednisolone regimens. A 5-year study. Int J Dermatol 1990;29:363-7.
[Google Scholar]
|
| 11. |
Strowd LC, Taylor SL, Jorizzo JL, Namazi MR. Therapeutic ladder for pemphigus vulgaris: Emphasis on achieving complete remission. J Am Acad Dermatol 2011;64:490-4.
[Google Scholar]
|
| 12. |
Seidenbaum M, David M, Sandbank M. The course and prognosis of pemphigus. A review of 115 patients. Int J Dermatol 1988;27:580-4.
[Google Scholar]
|
| 13. |
Olszewska M. Risk of disease relapse after treatment discontinuation in pemphigus vulgaris. J Am Acad Dermatol 2008;58:AB84.
[Google Scholar]
|
| 14. |
Kridin K, Sagi SZ, Bergman R. Mortality and cause of death in patients with pemphigus. Acta Derm Venereol 2017;97:607-11.
[Google Scholar]
|
| 15. |
Ljubojević S, Lipozencić J, Brenner S, Budimcić D. Pemphigus vulgaris: A review of treatment over a 19-year period. J Eur Acad Dermatol Venereol 2002;16:599-603.
[Google Scholar]
|
Fulltext Views
3,363
PDF downloads
1,487





