Translate this page into:
Clinico-epidemiological profile of reactive arthritis patients attending tertiary care center of Eastern India
Corresponding author: Dr. Swetalina Pradhan, Department of Dermatology, All India Institute of Medical Sciences, Patna, Bihar, India. dr.swetalinapradhan@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Pradhan S, Shahid R. Clinico-epidemiological profile of reactive arthritis patients attending tertiary care center of Eastern India. Indian J Dermatol Venereol Leprol. 2025;91:S13-S20. doi: 10.25259/IJDVL_1189_2023
Dear Editor
Reactive arthritis (ReA) is a conglomeration of articular, entheseal, mucocutaneous, and ocular manifestations resulting from an autoimmune response to gastrointestinal (GI) infections with Shigella, Salmonella, Campylobacter, and other organisms, as well as genitourinary (GU) infections (especially with Chlamydia trachomatis).1 The diagnosis of ReA is mostly clinical because of the unavailability of definitive diagnostic tests or validated criteria.2 The Indian published data on ReA primarily comes from rheumatology, with a paucity of data from dermatology.
This retrospective study evaluated 64 ReA patients treated in the Dermatology department from January 2019 to January 2023, following approval from the Institute Ethical Committee. Patient history, demographic data, mucocutaneous manifestations, joint involvement, associated systemic manifestations, laboratory parameters [complete blood count, liver function tests, renal function tests, viral markers (HIV, HBsAg, HCV)] and treatment received were extracted from the in-patient files. Diagnosis of ReA was made based on the presence of: (1) arthritis (asymmetric, mono, or oligoarthritis), (2) muco-cutaneous manifestations, (3) preceding history of enteritis (diarrhoea for at least 1-3 days to 6 weeks before the onset of arthritis) or urethritis (dysuria or discharge for at least 1-3 days to 6 weeks before the onset of arthritis), (4) positive HLA B27, (5) elevated erythrocyte sedimentation rate (ESR), and C-reactive protein (CRP). Urine cultures were performed in all, potassium hydroxide microscopy and wet mount in patients with active urethral discharge, and X-ray of the involved joints were taken. Nucleic acid amplification test (NAAT) and polymerase chain reaction (PCR) could not be performed due to the lack of facilities.
Patients with active urethral discharge received doxycycline 100 mg twice a day for 3 weeks. Various treatments offered for ReA included biologics (injection infliximab, etanercept, and secukinumab), dexamethasone methotrexate pulse (DMP), oral indomethacin/tramadol, oral methotrexate, and sulfasalazine. Those willing to take injectables received either DMP or biologics, based on affordability. For DMP, patients received 100 mg of dexamethasone intravenously for 3 consecutive days at a 28-day interval, along with weekly methotrexate (15–20 mg) and daily sulfasalazine. Infliximab injection was administered as a 200 mg IV infusion at 0, 2, and 6 weeks, followed by a maintenance dose every 8 weeks. Secukinumab was given as a 300 mg subcutaneous injection at weeks 0, 1, 2, 3, 4, then every 4 weeks. Etanercept was administered as a 50 mg subcutaneous injection twice weekly for 3 months, then 50 mg weekly. All patients on biologics also received weekly methotrexate (10–20 mg), except one patient who was HbsAg positive. The Visual Analogue Scale (VAS) was used to assess improvement in joint pain and skin lesions. Score zero represented no improvement (at baseline), whereas score 10 was taken as a complete improvement for both joint pain and skin lesions.
Statistical analysis was performed using SPSS version 22 software. Frequencies and percentages were used for categorical variables, while mean and standard deviation were used for numerical variables. Treatment duration was modelled to calculate the mean VAS score improvement in joint pain and skin lesions for both treatment groups (DMP vs. infliximab). The treatment duration-weighted mean VAS scores between the two groups were compared using an unpaired “t” test.
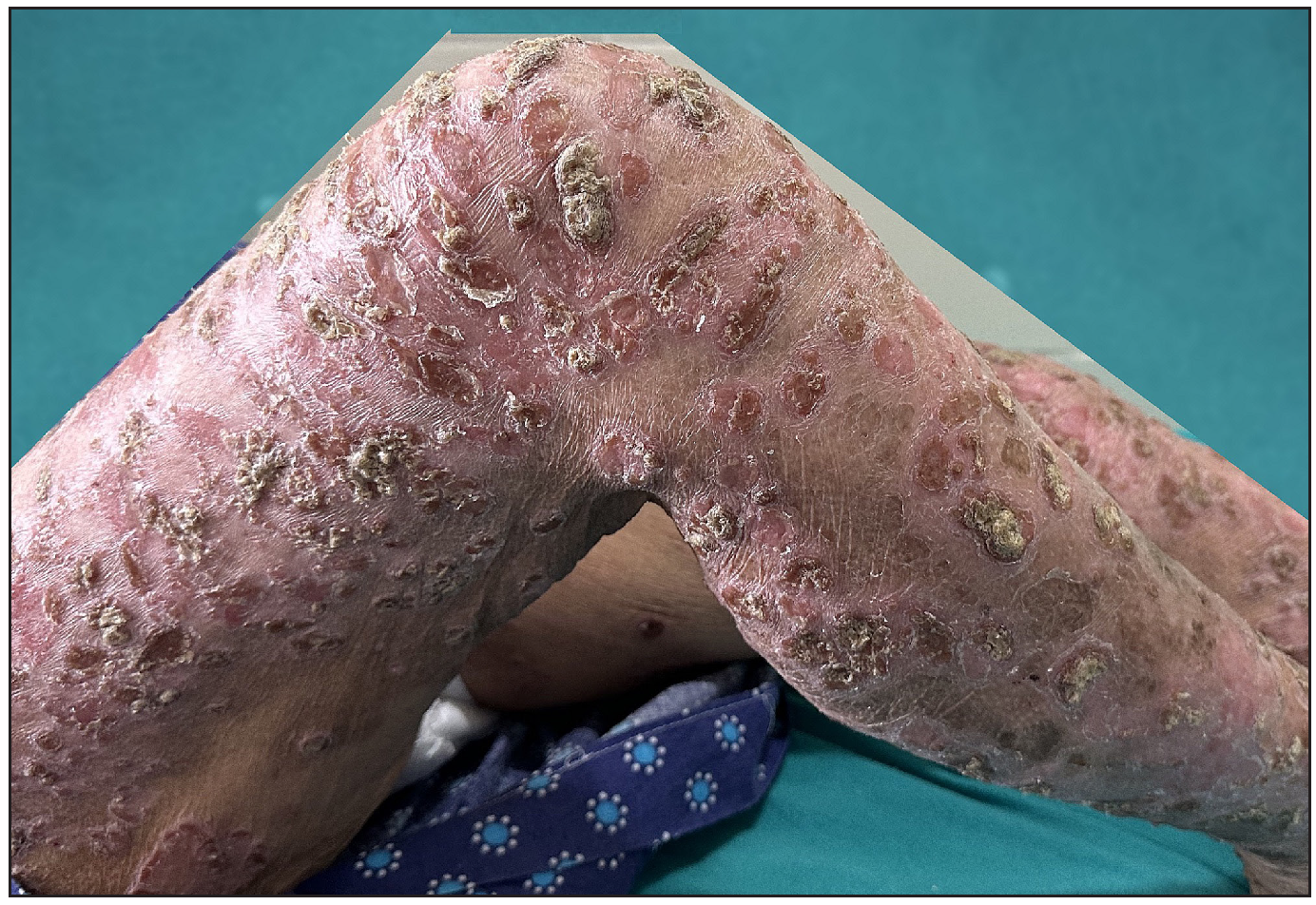
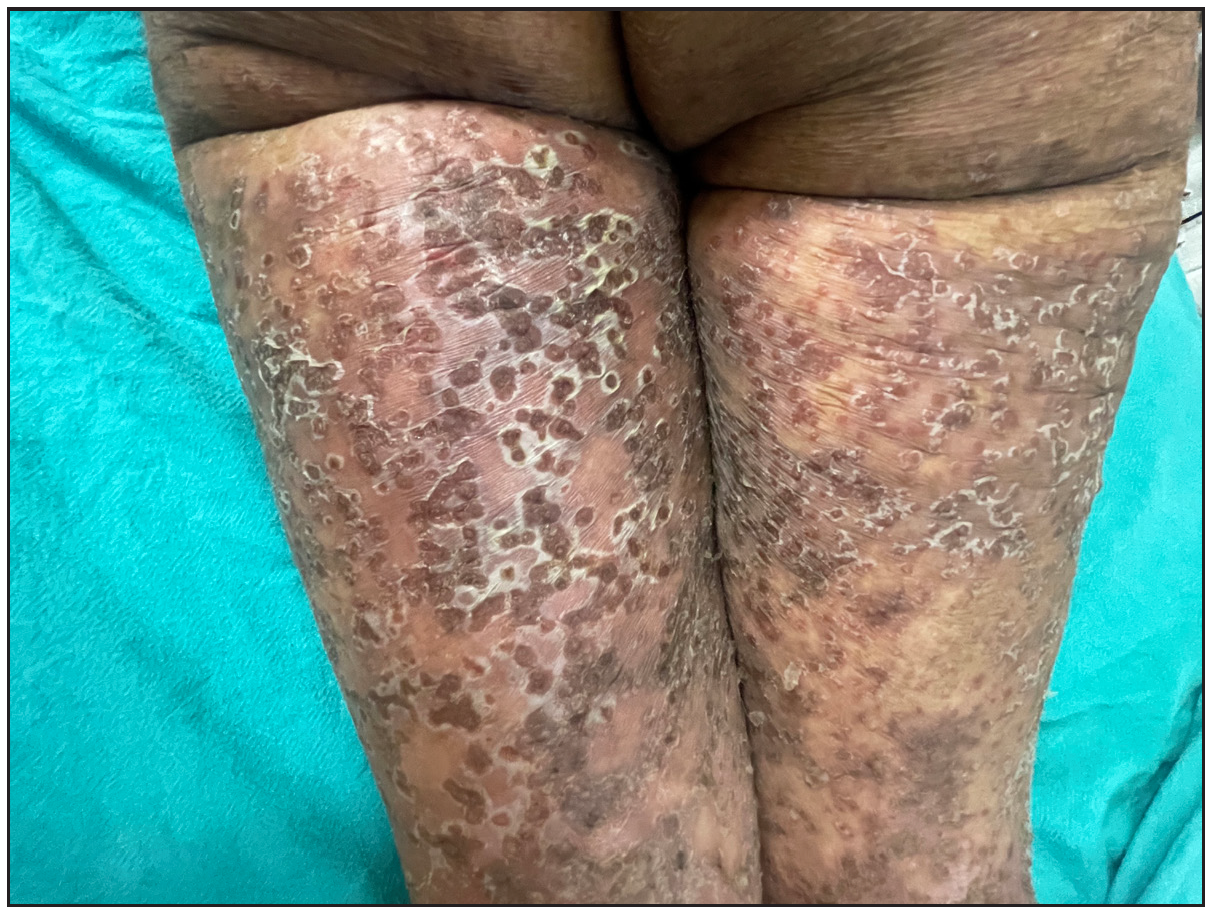
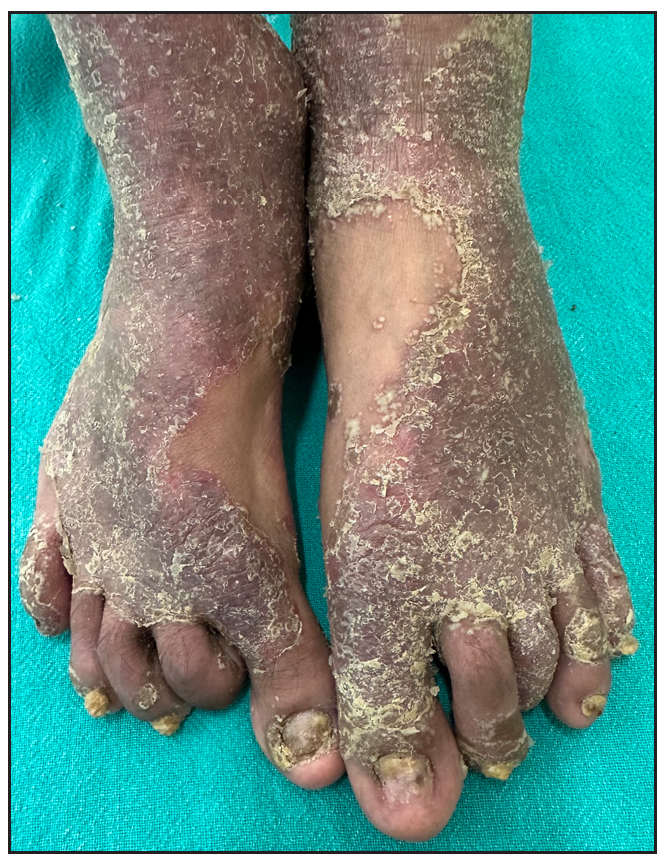
Out of a total of 2127 admitted patients, 64 (3.01%) had ReA. The socio-demographic details are given in Table 1. Muco-cutaneous lesions preceded joint involvement in 11 cases (17.1%), while 14 cases (21.8%) had simultaneous involvement, while in 39 cases (60.9%), joint pain occurred first. The primary skin lesions were either papules (53-82.8% cases) or pustules (21-32.8% cases) [Figures 1a,1b and 2a, 2b]. Both types of lesions evolved into crusted papules with a peripheral collarette of scales [Figures 1a and 1b], which were subsequently shed, leaving residual hyper/hypopigmentation. Plaques formed by the coalescing of crusted papules had collarettes of scales [Figures 2a and 2b]. Other findings included annular plaques [Figure 3a-3c], keratoderma blenorrhagica, crusted plaques on the scalp [Figures 4a and 4b], circinate plaques in the axilla and inguinal areas [Figures 5a and 5b], circinate balanitis, and circinate vaginitis [Figures 6a and 6b]. Table 2 enumerates the muco-cutaneous manifestations. Sacroiliac joint was the most commonly involved joint, with 34 cases (53.12%) having some form of joint deformity [Table 3] [Figure 7]. Recurrent conjunctivitis was documented in 23 cases (35.9%). Renal and liver involvement were detected in 3 (4.6%) and 4 (6.2%) cases respectively.
| Socio-demographic characteristics | Number and percentage |
|---|---|
| Mean age | 35.18 + 11.2 .53 years |
| Peak age of disease onset | 20-40 years |
| Male/female | 55 (85.9%), 9 (14.1%) |
| Rural/Urban | 53 (82.8%), 11 (17.2%) |
| Married/unmarried | 43 (67.2%), 21 (32.8%) |
| Low socioeconomic background | 21 (32.8%) |
| Multiple sex partners | 36 (56.2%) |
| Addiction to tobacco and alcohol | 37 (57.8%) |
| Average duration of disease | 2.2 years (4 months-420 months) |
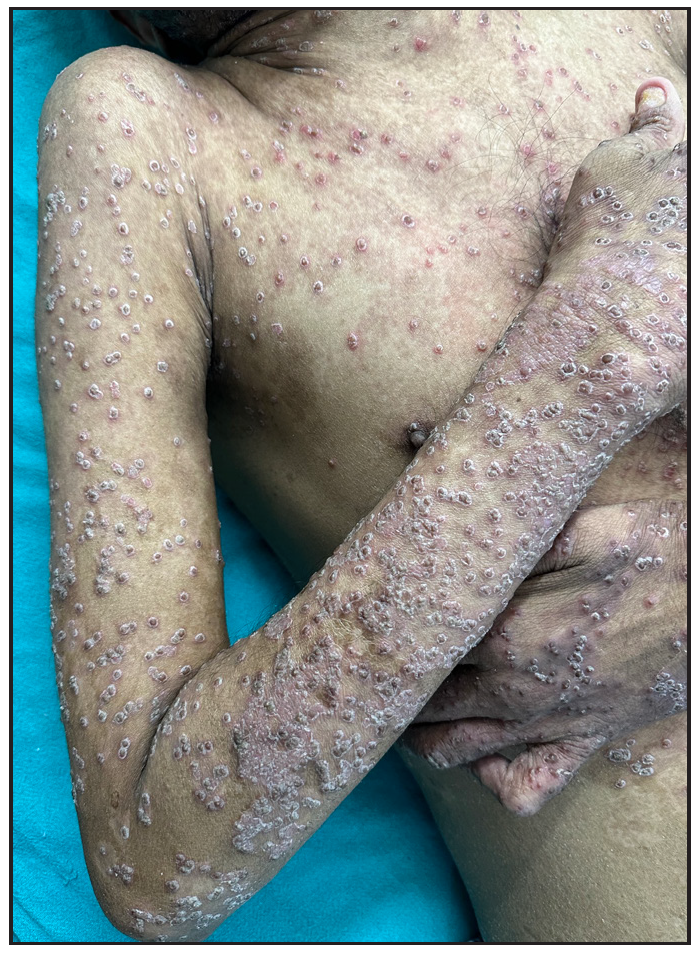
- Multiple papules, crusted papules with collarette of scales.
- Crusted plaques with collarette of scale.
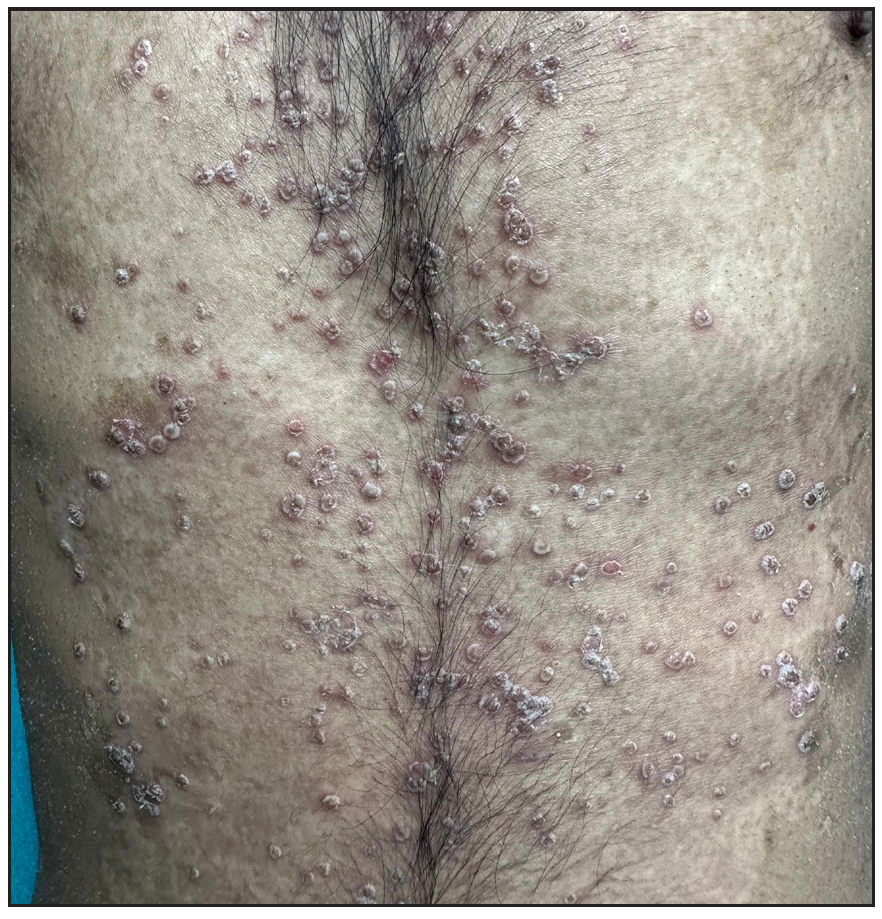
- Multiple pustules over the trunk.
- Crusted plaques with collarette of pustules.
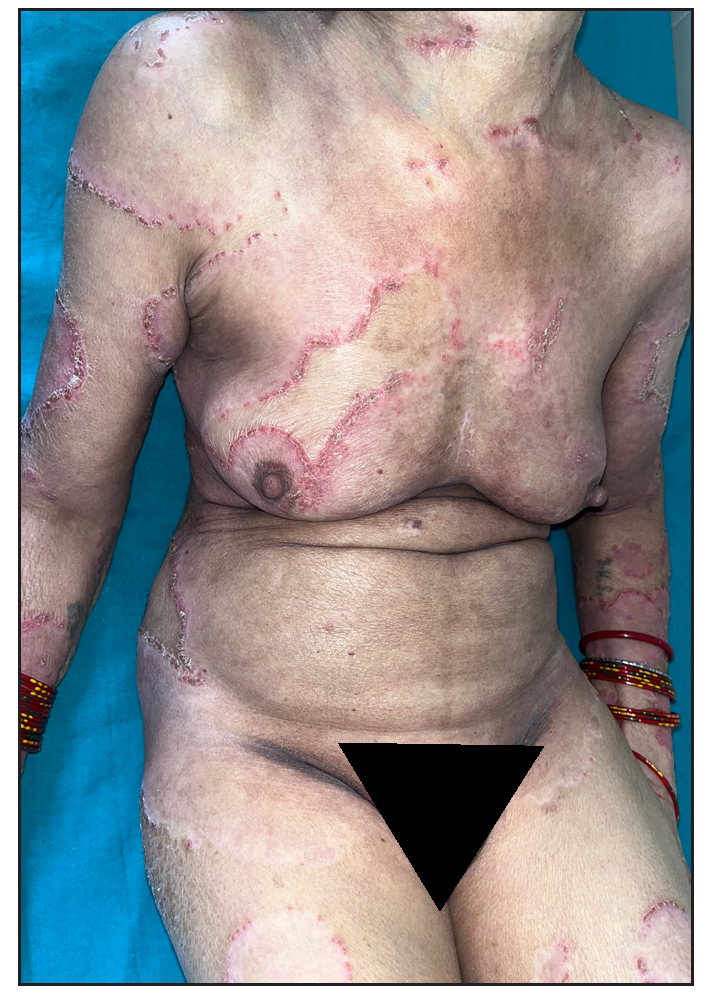
- Multiple large annular plaques with crusted papules at margin in a female.
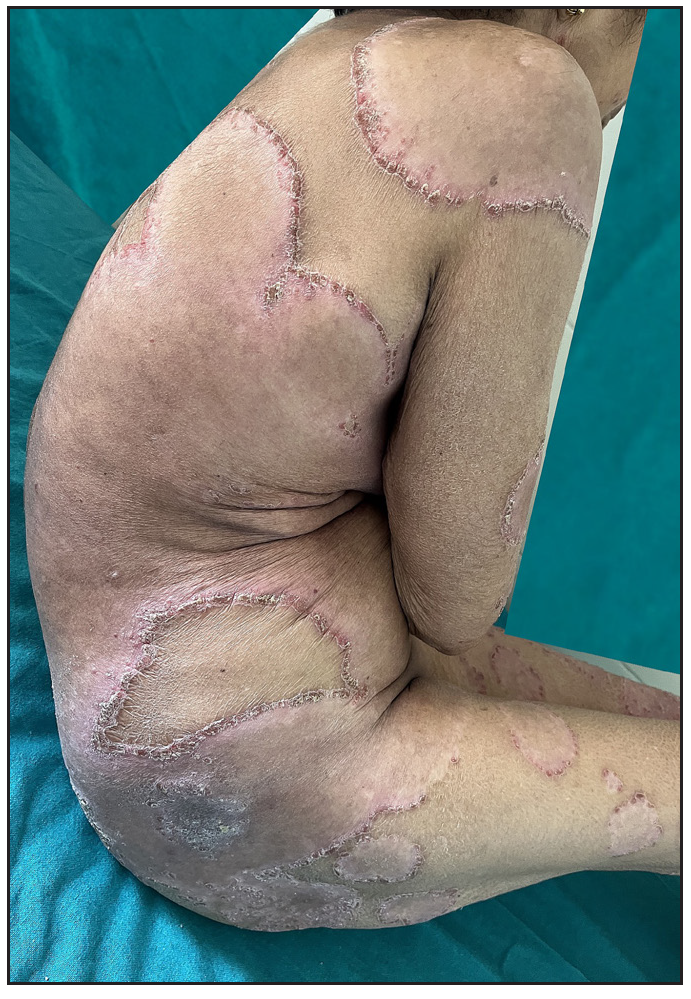
- Multiple large annular plaques with crusted papules at margin in a female.
- Complete improvement of skin lesions post infliximab injection.
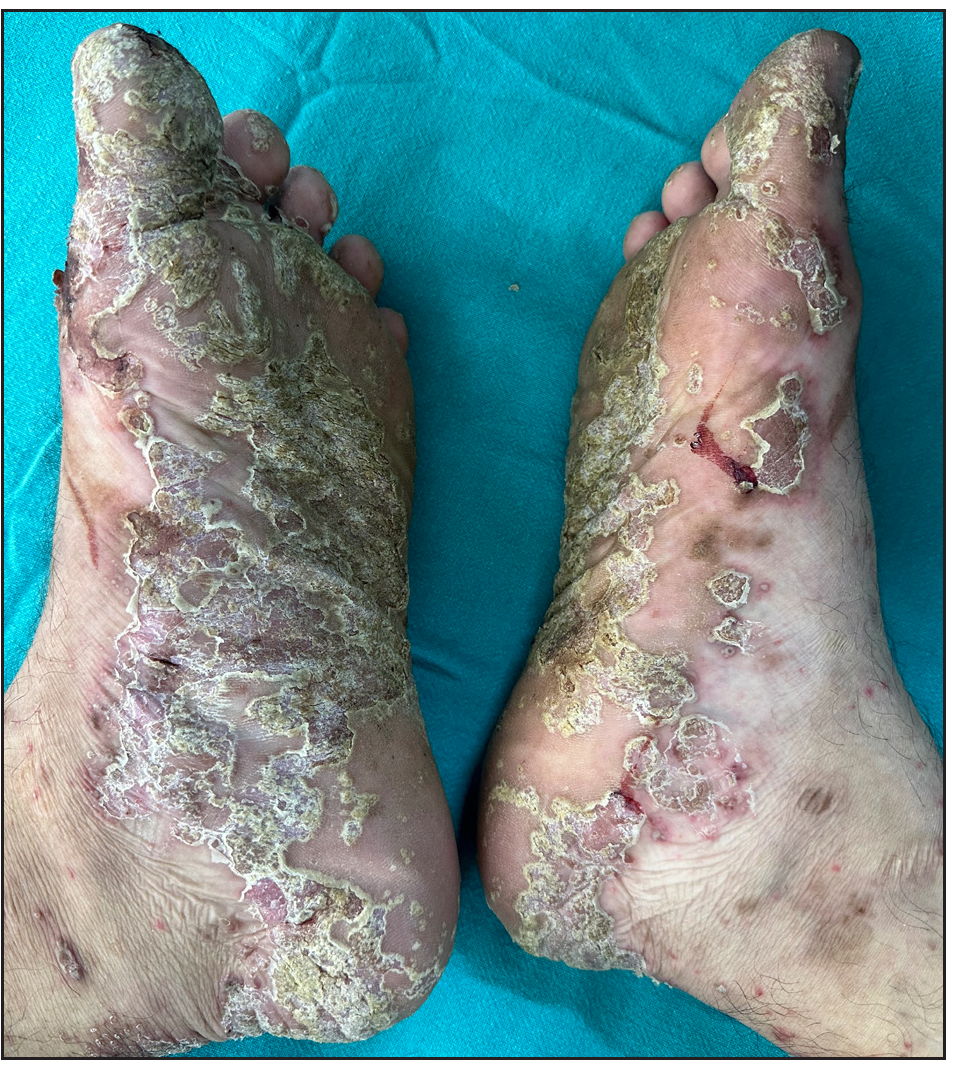
- Keratoderma blenorrhagica over soles.
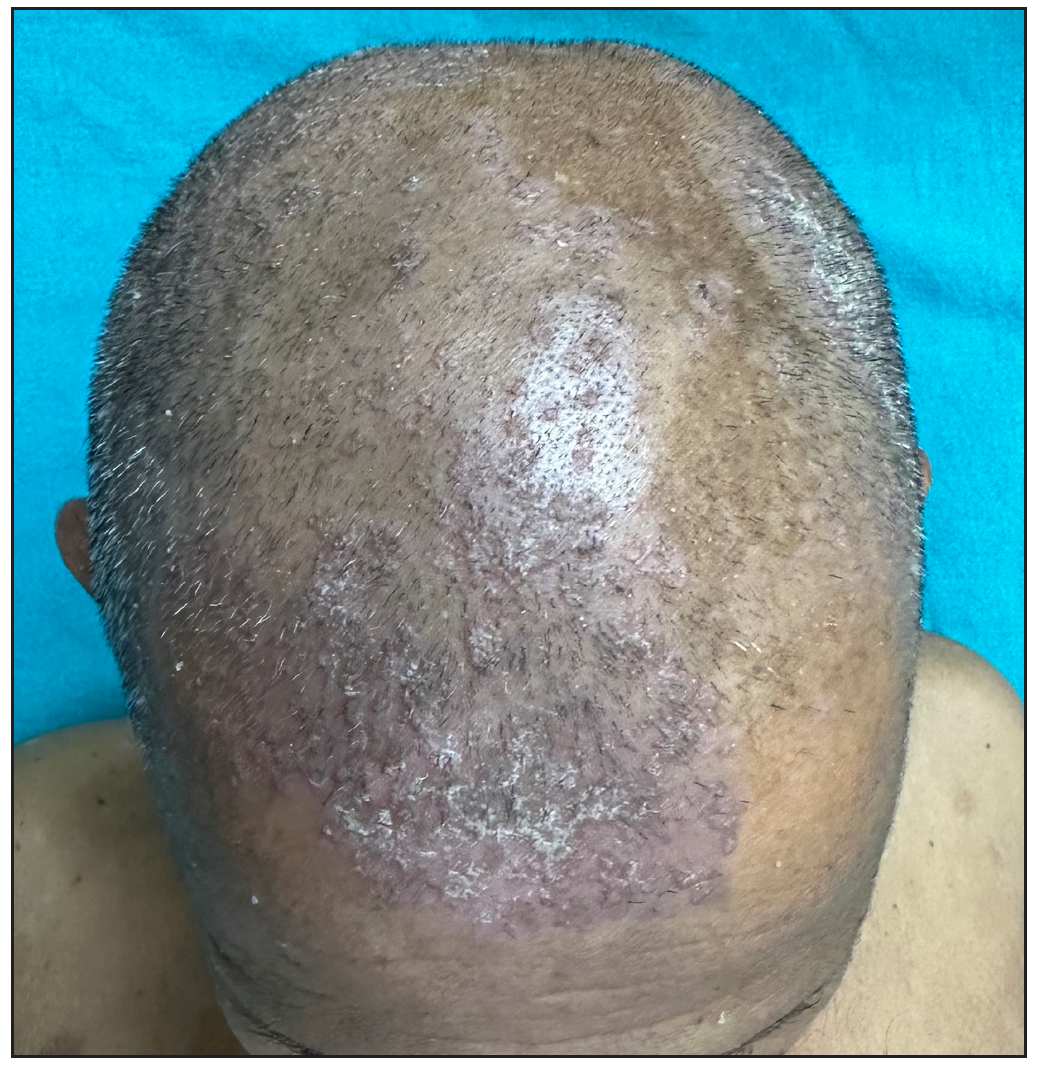
- Crusted papules and plaques over scalp.
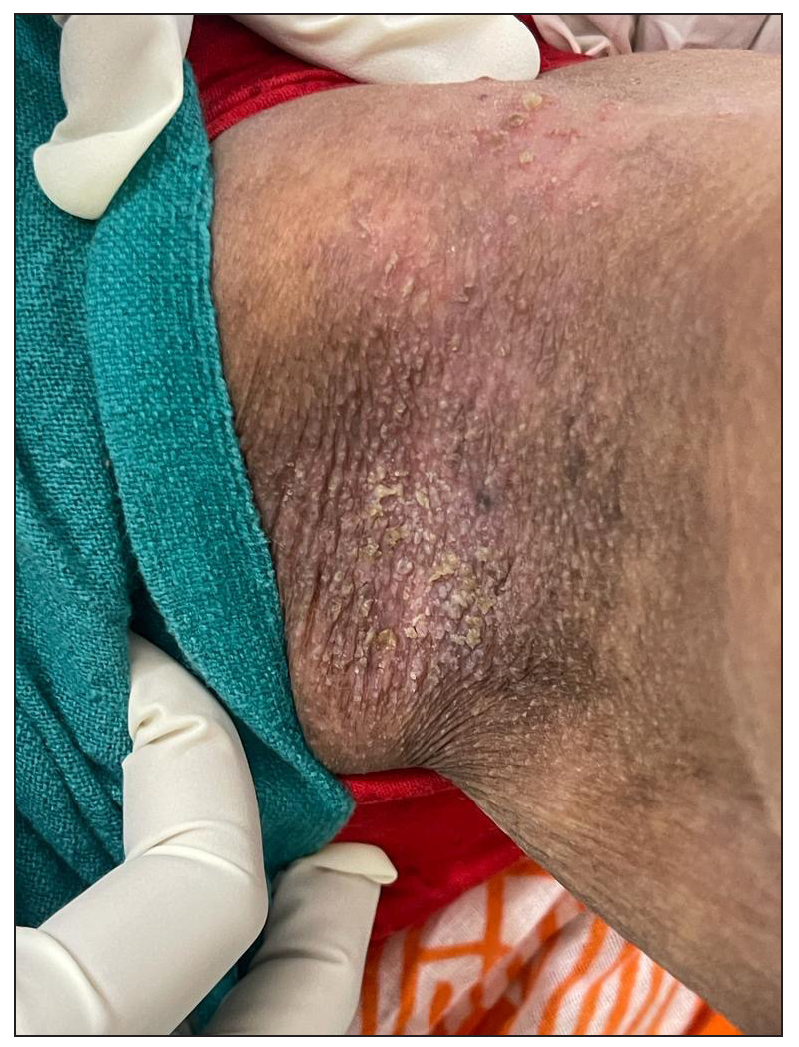
- Maceration and circinate plaques in axilla.
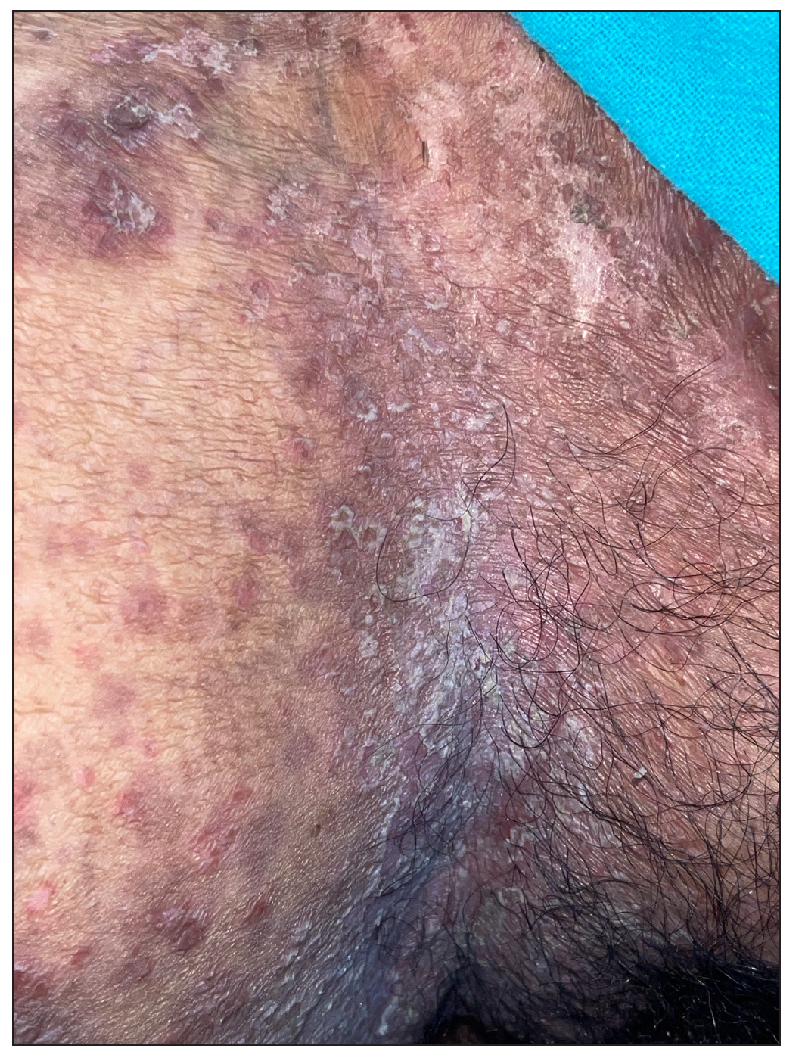
- Circinate plaques over inguinal area.
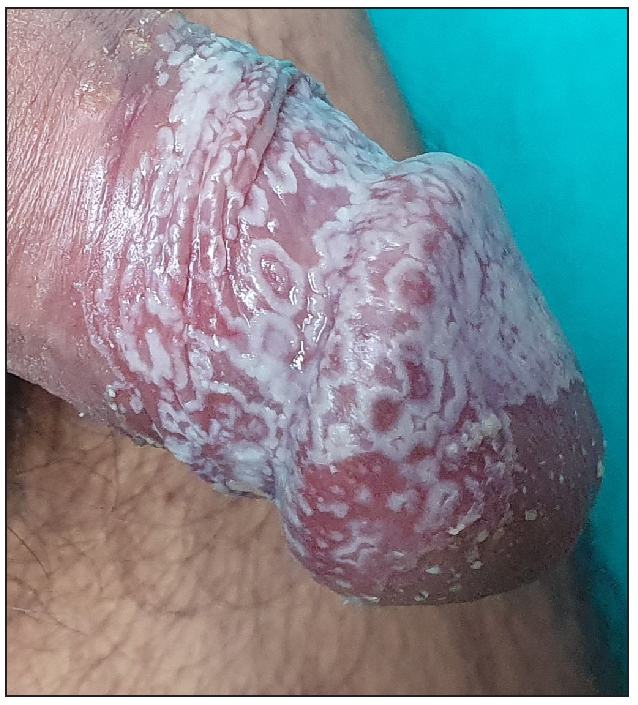
- Circinate balanitis.
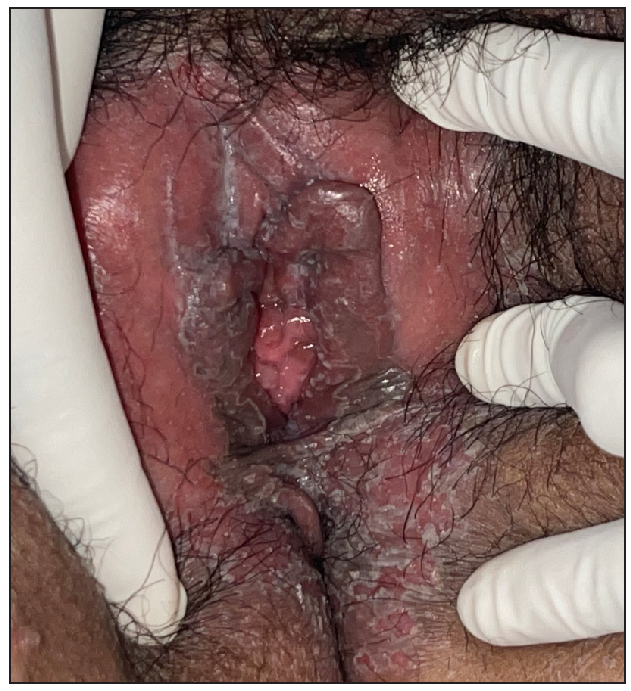
- Circinate vaginitis.
| Type of lesions | Number (%) |
|---|---|
| Papules | 53 (82.8%) |
| Crusted papules | 64 (100 %) |
| Crusted plaques with collarette of scale | 64 (100 %) |
| Pustules | 21(32.8%) |
| Annular plaques, Circinate plaques over flexures | 4 (6.2%) each |
| Crusted papules and plaques on scalp | 19 (29.7%) |
| Keratoderma blenorrhagica in palms and soles | 20 (31.2%) |
| Aphthous like an oral ulcer | 3 (4.6%) |
| Circinate balanitis | 34 (53.1%) |
| Circinate vaginitis | 4 (6.2%) |
|
Nail changes -Total Total nail dystrophy Nail plate thickening with subungual hyperkeratosis Distal onycholysis Nail fold inflammation |
36, 56.25% 19, 29.6% 23, 35.9% 29, 45.3% 11, 17.1% |
| Type of joint | Number (%) |
|---|---|
| Sacroiliac | 33 (51.6 %) |
| Knee | 29 (45.3%) |
| Ankle | 23 (35.9%) |
| Cervical | 12 (18.7%) |
| Elbow, | 11 (17.2%) |
| Wrist | 11 (17.2%) |
| Small joints of hands | 11 (17.2%) |
| Small joints of feet | 5 (7.8%) |
| Deformities | 34 (53.1%) |
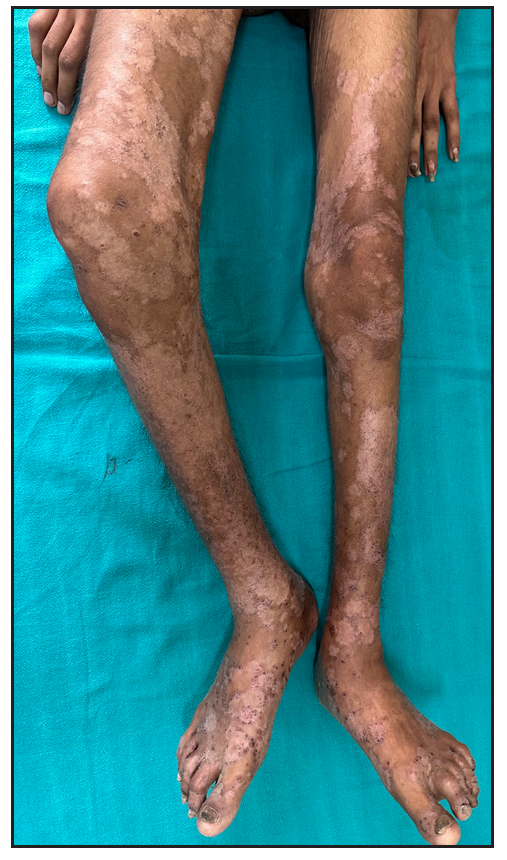
- Patient with contracture of right knee.
- Flexion deformity of toes with nail dystrophy and nail fold involvement.
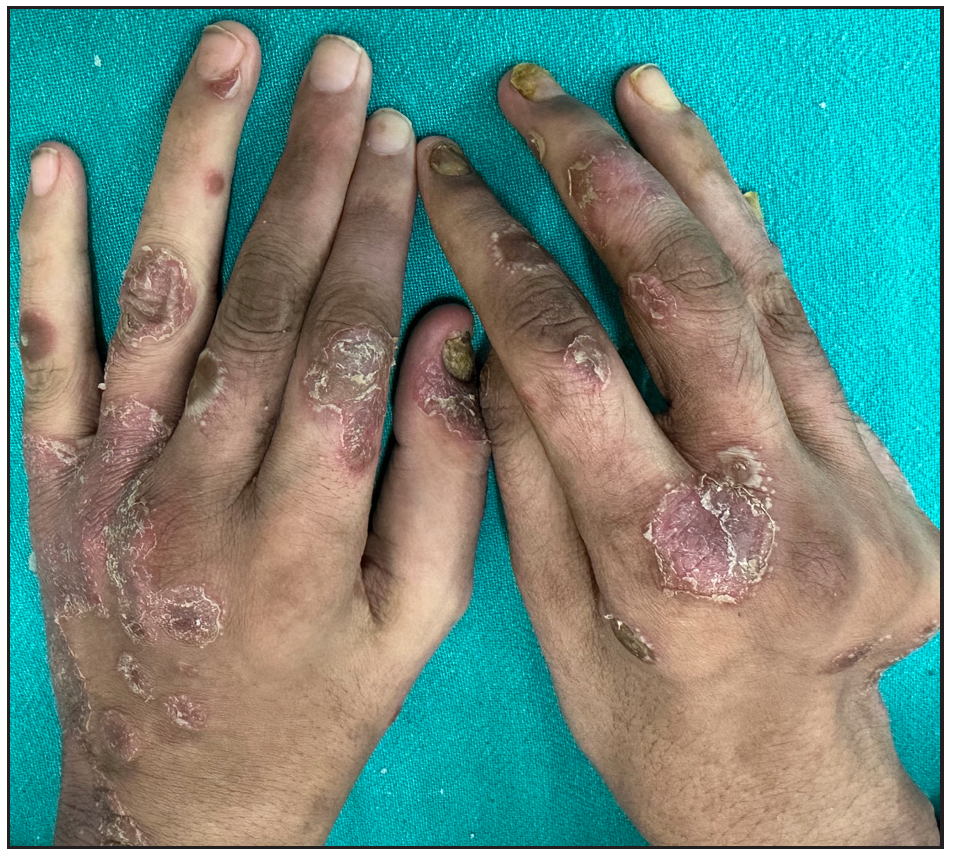
- Deformity at metacarpophalangeal joint.
All cases had elevated ESR and CRP, with mean values of 81.07 + 20.52 and 114.65 + 56.26, respectively. Two cases tested seropositive for HIV and one for HbsAg. Gram stain was negative for diplococci in all 27 cases with active urethral discharge, and 6 cases had >2 WBC per oil immersion field. KOH mount was negative and urine culture was sterile in all cases. Four cases had elevated liver transaminases. Elevated urea and creatinine, as well as high-grade proteinuria, were found in 3 and 2 cases, respectively. X-ray findings included reduced to absent joint space, juxta-articular osteopenia, enthesopathy, sacroiliitis, bony ankylosis, and periarticular erosions [Figures 8a-8d].
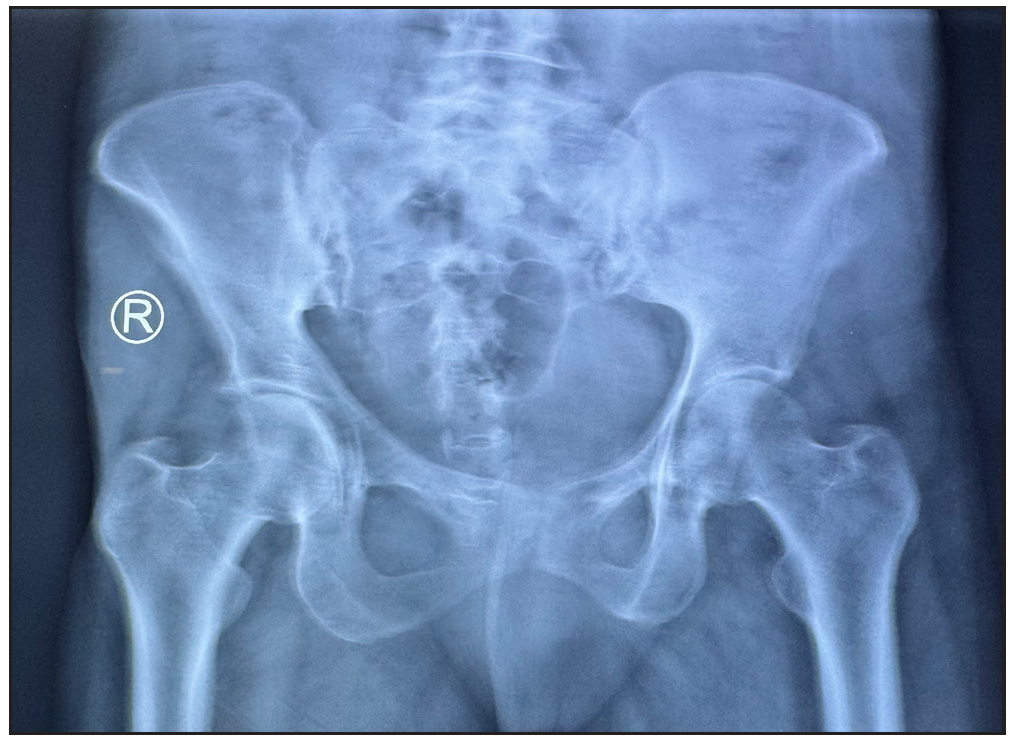
- X-ray pelvis shows subchondral sclerosis with articular margin irregularity in right sacroiliac joint suggestive of sacroiliitis.
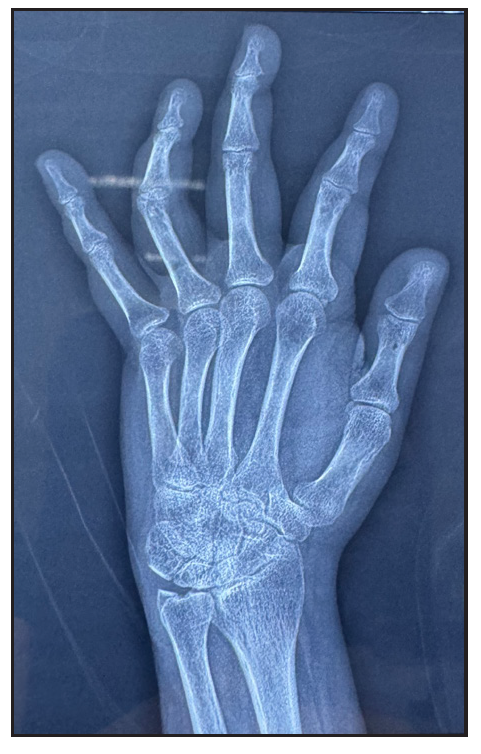
- X-ray of left hand shows reduced radio-carpal joint space with juxta-articular osteopenia at wrist joint, intercarpal joints, metacarpophalangeal joints and interphalangeal joints.
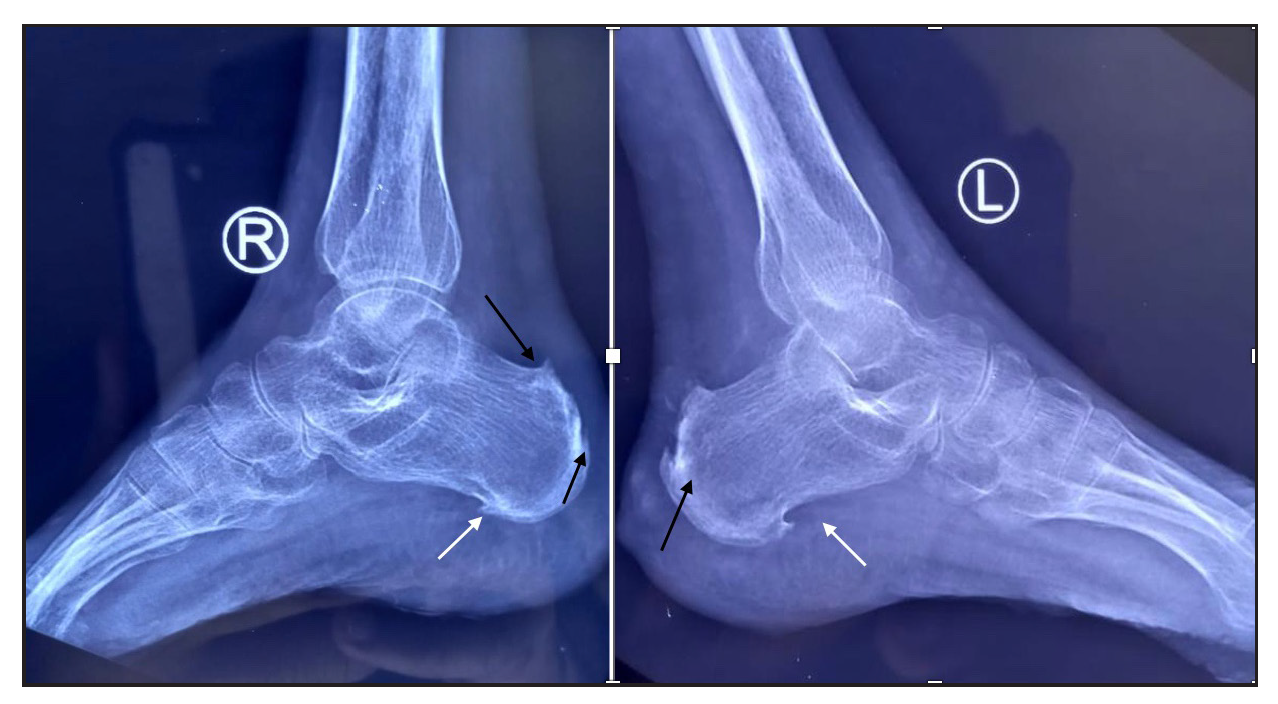
- X-ray of bilateral ankle joint shows plantar calcaneal spur (white arrows) and dorsal calcaneal spur at Achilles tendon insertion (black arrow) suggestive of enthesopathy.
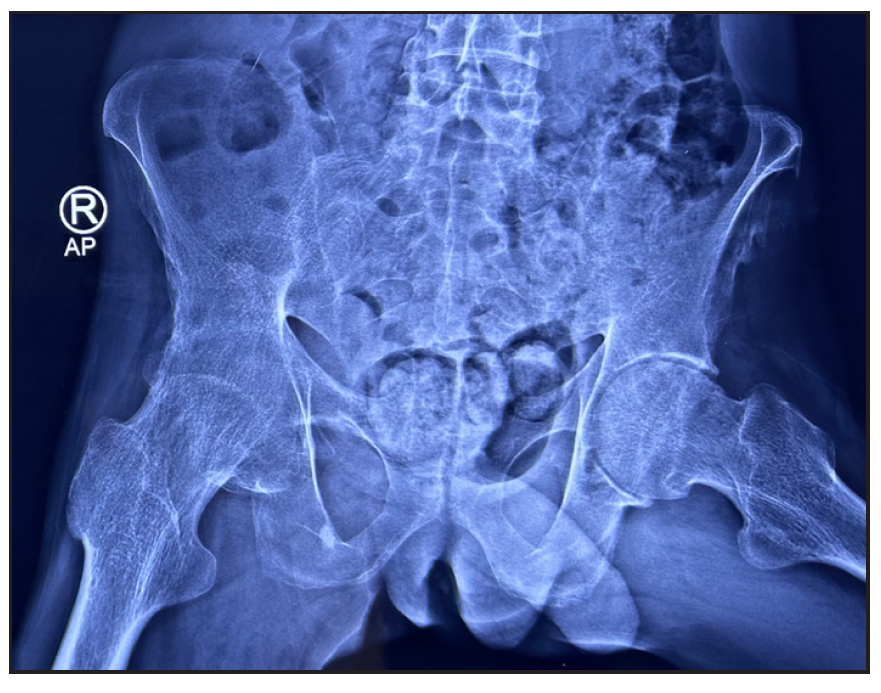
- X-ray of the pelvis shows irregularity of left ischial tuberosity with new bone proliferation suggestive of enthesopathy.
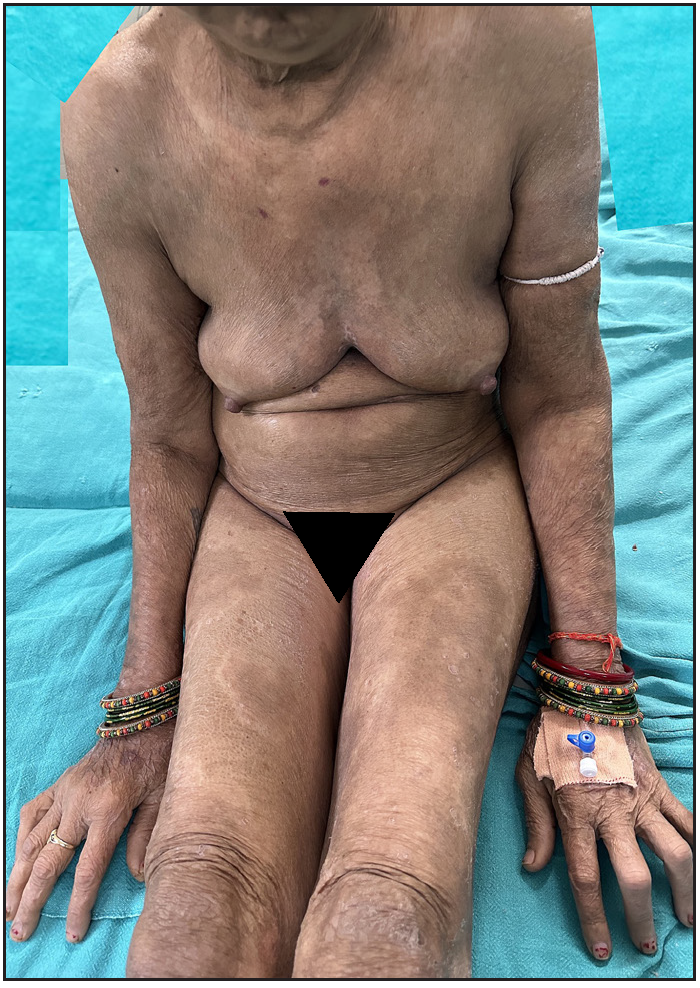
Of 64 patients, 28 (43.7%) received dexamethasone methotrexate pulse (DMP), 32 (50%) received biologics, and 4 (6.2%) were on a combination of methotrexate (MTX) and sulfasalazine combination therapy. Infliximab was administered to 30 patients, one HbsAg-positive patient received a secukinumab injection, and one patient received an etanercept injection. The mean treatment duration for all patients, DMP group and infliximab group was 8.07 + 6.13 months, 3.67 + 2.16 months, and 12.95 + 5.57 months, respectively. The treatment duration-weighted mean VAS score improvement in joint pain was found to be significantly higher among patients receiving infliximab than patients receiving DMP [(8.14 +1.24) vs (2.56 +1.27); p-value = 0.000]. Similarly, the treatment duration-weighted mean VAS score improvement in skin lesions was found to be significantly higher among patients receiving infliximab than patients receiving DMP [9.01 +1.10) vs. 3.53 +1.60); p-value = 0.000]. Complete improvement in skin lesions and joint pain was observed in 19 (29.7%) and 4 (6.3%) patients, respectively, with injection infliximab [Figure 9a and 9b]. There was a complete improvement in nail dystrophy in five cases with infliximab injection [Figure 9c and 9d]. A significant improvement in joint pain and skin lesions was found with secukinumab but there was recurrence 6-weeks after treatment discontinuation. With etanercept also, significant improvement in skin lesions and joint pain was observed during a 4-month treatment period. There were no side effects to treatment with biologics. In the DMP group, two patients developed hyperglycaemia after three cycles and one patient complained of blurring of vision after 6 pulses.
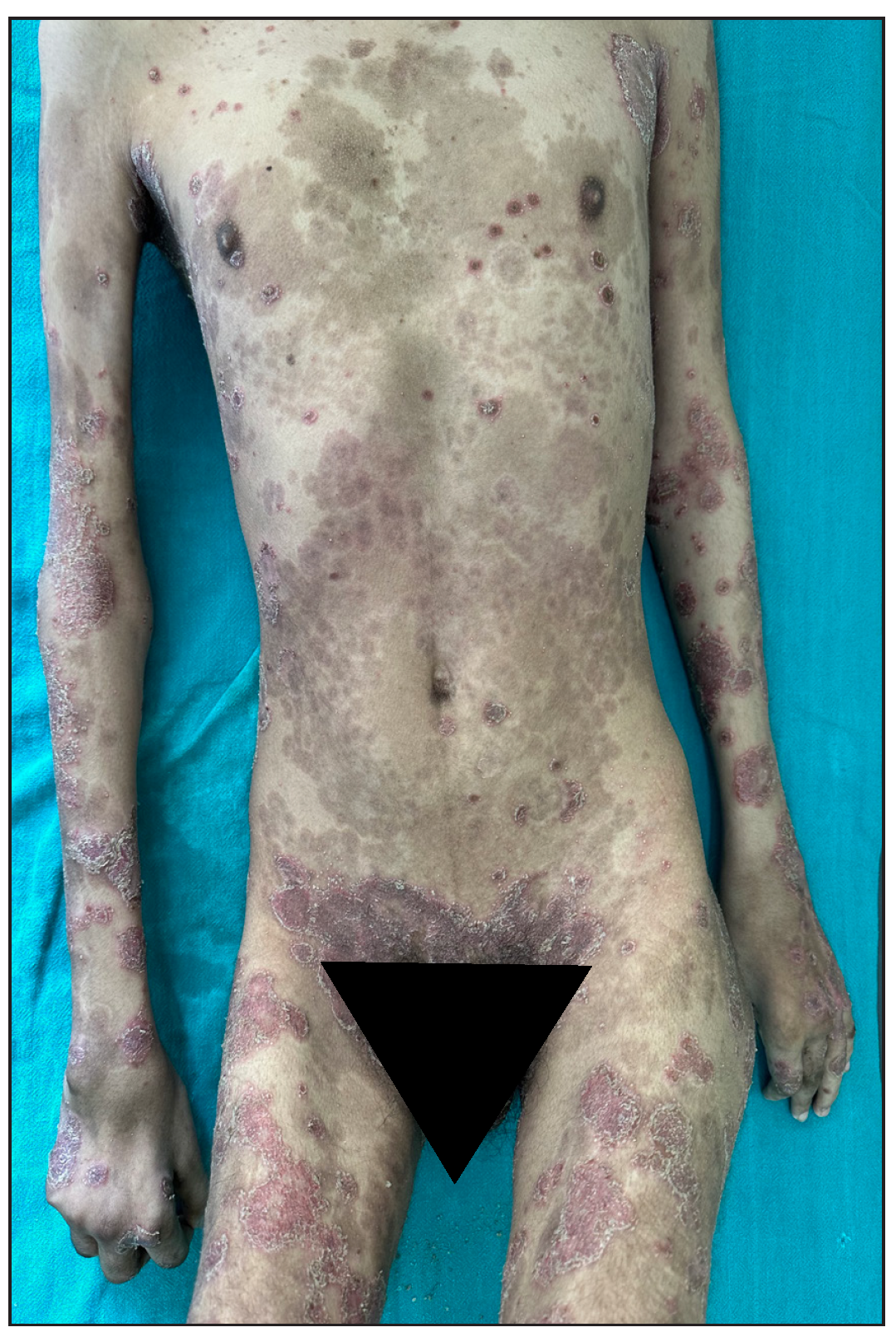
- Pre-infliximab baseline picture of skin lesions loading dose.
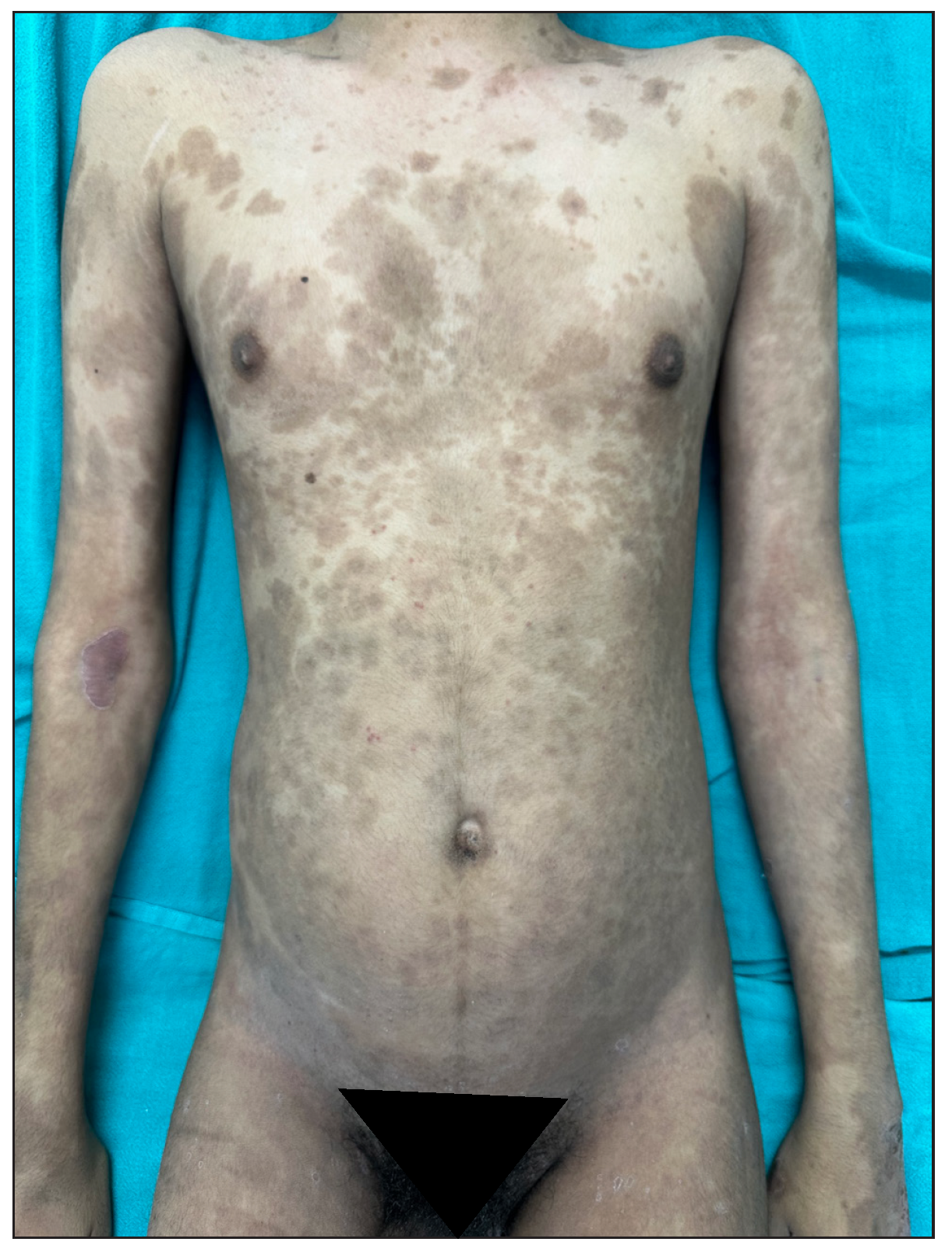
- Complete improvement of skin lesions post-infliximab 8 weeks.
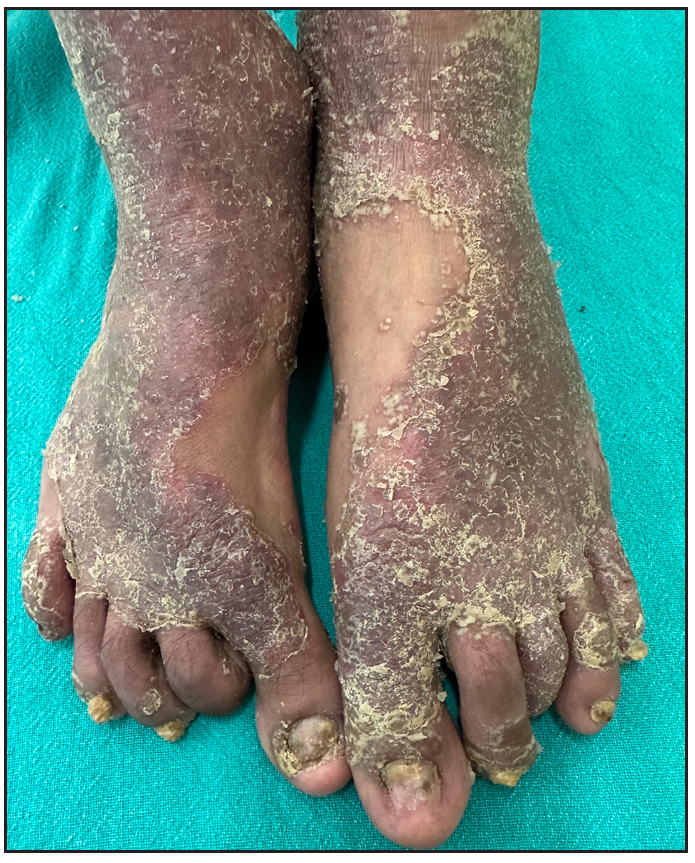
- Pre-infliximab baseline picture of nail dystrophy.
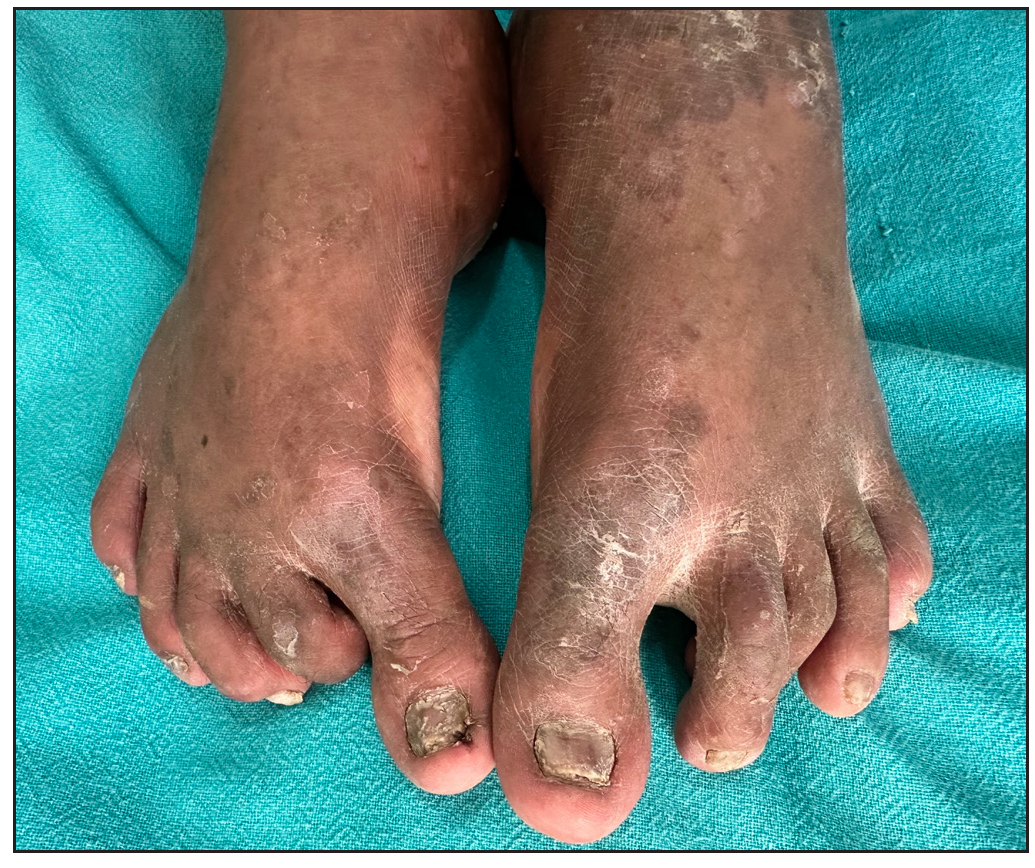
- Improvement in nail dystrophy post-infliximab 5 months post treatment.
In the United States, the frequency of ReA ranges from 3.5 to 5 patients per 100,000, which is comparable to our study.3 Demographic and clinical profiles of patients were also similar to those in other studies [Table 4].4-6 Methotrexate has been the most commonly used DMARD for treating chronic ReA, but has proved to be ineffective in clinical trials.7 Table 5 details the outcomes of biologicals in ReA. Pasricha was the first to try dexamethasone pulse therapy for ReA, with encouraging results. Kanwar et al. reported good results, including rapid relief of symptoms in 2 patients in their study.13 Dexamethasone pulse therapy is frequently practiced in India to treat reactive arthritis and can serve as a more affordable alternative for patients unable to afford biologics.
| Variables | Thomas et al.4 | Gupta et al.5 | Lahu et al.6 | Current study |
|---|---|---|---|---|
| Peak age group (years) | 20-40 | |||
| Male: Female | 5.8:1 | All males | 1.9:1 | 6.1:1 |
| Disease duration | 4 (2–12) years | 9-180 months | - | 2.22 years (4 months-420 months) |
| Joints affected | Knee (88%) ankle (38%), midtarsal, elbow, wrist (10%), and small joints of the hands (2%–4%) |
Knees (91.6%), Sacro-iliac joints (66.6%), Elbows (66.6%), Wrists (66.6%), Ankles (58.3%), Interphalangeal joints (50%) and Shoulders (33.3%) |
Knee joint was the most affected (M=52.12%, F= 64.7%) followed by talocrural (T/C) articulation (M=57.57%, F=50%), metatarsophalangeal (MTPH) articulation (M=48.48%, F=41.11%), radiocarpal (R/C) articulation (M=48.48%, F=44.11%) |
Sacroiliac 51.56%, Knee 45.31%, ankle 35.93%, cervical 18.75%, elbow 17.18%, wrist 17.18%, small joints of hans 17.18%, small joints of feet 7.81% |
| Mucocutaneous findings | oral ulcers (6.5%), psoriasiform skin lesions (4.8%), and genital ulcers (1.6%) | rupioid plaques 100%, peripheral pustulation (33%), Keratoderma blenorrhagicum 42%, circinate balanitis (25%) | - | Papules (100%), crusted papules (100%), pustules 14.06%, crusted plaques with a collarette of scales (100%), annular plaques (6.2%) crusted plaques on scalp (29.7%), oral ulcer (4.6%), keratoderma blenorrhagicum (31.25%), circinate plaques over flexural areas (4.6%) and circinate balanitis (53.1%) |
| Nail changes | - | nail plate thickening (83.3%), distal onycholysis(83.3%), and subungual hyperkeratosis (8.3%) | - | Total nail dystrophy (29.6%), nail plate thickening with subungual deposits (35.9%), distal onycholysis (45.3%), nail fold inflammation (17.1%) |
| Eye involvement | Uveitis 5.8% |
Conjunctivitis (8.3%) Healed Uveitis (8.3%) |
- | Conjunctivitis (35.9%) |
| Publication type, number of patients | Biological, Dose and duration | Outcome of arthritis | Outcome of skin lesions |
|---|---|---|---|
|
Brief report, Meyer et al.,8 5 patients |
Infliximab 4 patients 5mg/kg and 1 patient 3mg/kg infliximab Follow up duration for 12 to 50 months |
After 6 months significant improvement in arthritis with zero swollen and tender joints | - |
|
Case report, Gill H, et al.,9 1 patient |
Infliximab 200 mg intravenously at weeks 0, 2, 6, and 14 weeks |
complete resolution of arthritis within 6 weeks of infliximab therapy. | complete resolution of skin lesions within 6 weeks of infliximab therapy |
|
Case report, Gaylis N et al.,10 1 patient HIV positive |
Infliximab 300 mg intravenously at Weeks 0, 2, and 6 and then every 6 to 7 weeks thereafter |
After 6 months taking infliximab, arthritis resolved, no change in viral load | After 6 months taking infliximab, nails regrew, and the rash cleared |
|
Case report, Padhan P et al.,11 1 patient |
Secukinumab 150 mg subcutaneously weekly for 4 weeks followed by monthly for 12 months |
Asymptomatic after 3 months | - |
|
6 month open-label study, Flagg SD, et al.,12 7 patients with ReA among total of 16 patients |
Etanercept 25 mg subcutaneously twice weekly, for 6 months | Majority showed clinical, and functional improvement | - |
|
Current study Total patients on biological 32 Infiximab-30, Secukinumab-1 Etanercept-1 |
Infliximab 200 mg IV infusion at 0, 2, and 6 weeks, followed by a maintenance dose at every 8 weeks intervalAverage duration of treatment: 12.95 + 5.57 months Secukinumab 300 mg subcutaneous was given at weeks 0, 1, 2, 3, 4 then every 4 weeks for 4 months Etanercept 50 mg subcutaneously twice weekly for 3 months, then 50 mg weekly for total 4 months |
Significant improvement in joint pain with complete improvement in 6.3% cases Significant improvement in joint pain during treatment recurrence post 6 weeks of discontinuation Significant improvement in joint pain during treatment and lost to follow up |
Significant improvement in skin lesions, nail with complete improvement of skin lesion in 29.7% cases and complete improvement in nail dystrophy in 5 cases Significant improvement in skin lesions during treatment and recurrence post 6 weeks of discontinuation Significant improvement in skin lesions during treatment |
Our study is the first from India to examine a large number of patients with ReA, focusing on muco-cutaneous manifestations and highlighting “new findings” such as annular plaques, circinate plaques over flexures, and vaginal mucosa in a few cases. We have described the response of skin lesions and arthritis to various treatment modalities, with a focus on the anti-TNF α drug Infliximab.
The drawback of our study is that it is a retrospective study, including only admitted cases with severe disease. Additionally, we did not perform culture or Nucleic acid amplification test (NAAT) in all cases.
Ethical approval
The research/study was approved by the Institutional Review Board at All India Institute of Medical Sciences Patna, number AIIMS/Pat/IEC|2}23 I 1061, dated 23/08/2023.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
References
- Reactive Arthritis. 2023 Jan 2. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023.
- [Google Scholar]
- An overview of reactive arthritis. J Am Acad Physician Assist.. 2019;32:25-8.
- [CrossRef] [Google Scholar]
- Clinical profile of adults and children with reactive arthritis in India – A cohort study. Indian J Rheumatol. 2020;15:304-9.
- [Google Scholar]
- A retrospective case series of 12 patients with chronic reactive arthritis with emphasis on treatment outcome with biologics. Indian J Dermatol Venereol Leprol. 2021;87:227-34.
- [CrossRef] [PubMed] [Google Scholar]
- Modes of presentation of reactive arthritis based on the affected joints. Med Arch.. 2015;69:42-5.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Safety and efficacy of anti-tumor necrosis factor α therapy in ten patients with recent-onset refractory reactive arthritis. Arthritis Rheum.. 2011;63:1274-80.
- [CrossRef] [PubMed] [Google Scholar]
- Successful use of infliximab in the treatment of Reiter’s syndrome: A case report and discussion. Clin Rheumatol.. 2008;27:121-3.
- [CrossRef] [PubMed] [Google Scholar]
- Infliximab in the treatment of an HIV positive patient with Reiter’s syndrome. J Rheumatol.. 2003;30:407-11.
- [PubMed] [Google Scholar]
- Secukinumab therapy in reactive arthritis: Report of two cases. Mod Rheumatol Case Rep. 2022;7(6):22-4.
- [CrossRef] [PubMed] [Google Scholar]
- Decreased pain and synovial inflammation after etanercept therapy in patients with reactive and undifferentiated arthritis: an open-label trail. Arthritis Rheum.. 2005;53:613-7.
- [CrossRef] [PubMed] [Google Scholar]
- Reactive arthritis in India: a dermatologists’ perspective. J Cutan Med Surg. 2013;17:180-8.
- [CrossRef] [PubMed] [Google Scholar]





