Translate this page into:
Clinicodermoscopic and immunopathological profile of non-infectious non-eczematous inflammatory tattoo reactions: A retrospective study from a tertiary care centre of East India
Corresponding author: Dr. Biswanath Behera, Department of Dermatology, AIIMS, Bhubaneswar, Odisha, India. biswanathbehera61@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Sethy M, Behera B, Dash S, Palit A, Nayak AK, Ayyanar P. Clinicodermoscopic and immunopathological profile of non-infectious non-eczematous inflammatory tattoo reactions: A retrospective study from a tertiary care centre of East India. Indian J Dermatol Venereol Leprol 2023;89:558–67.
Abstract
Introduction
Tattoo-associated complications are on the rise due to the popularity of decorative tattoos in recent years. The exact pathogeneses of various tattoo reaction patterns are still unclear, and their dermoscopic details are sparsely reported.
Aim
We aimed to retrospectively study the clinical, dermoscopic and immunopathological details of patients with non-infectious, non-eczematous inflammatory tattoo reaction patterns in a tertiary care centre of East India.
Method
The clinical, dermoscopic and pathological details of all the patients who had non-infectious, non-eczematous inflammatory tattoo reactions were collected. In all the cases, immunohistochemistry was done for CD1a, CD3, CD4, CD8, FoxP3, CD20 and CD56.
Results
A total of five patients of skin phototypes IV and V and six tattoo reactions were analysed. Five lesions had reactions at the site of a black tattoo, and one at the site of red tattoo. Clinically, the patients presented with erythematous or blue-grey flat-topped to verrucous papules and plaques. Dermoscopic features were dominated by a central white to pink-white structureless area, a peripheral grey-white to bluish-white structureless area, white scales, comedo-like opening with keratotic plugging, milia-like cysts and shiny white structures. Pathologically, except for one lesion that only showed a lichenoid reaction pattern in the red tattoo, all had a combination of reaction patterns. Immunohistochemistry showed increased epidermal and dermal Langerhans cells, predominantly CD8 positive T cells in the epidermis and dermis, sparse dermal B cells and CD4 positive T cells, reduced T regulatory cells and a complete absence of CD56 positive NK cells.
Limitations
Small sample size was the limitation of the study.
Conclusion
The clinical morphology and dermoscopy may not differentiate between various types of non-infectious non-eczematous inflammatory tattoo reactions. The immunological profile supports a delayed hypersensitivity reaction due to contact sensitisation to tattoo pigment, and CD8 positive T cells play a central role in executing various pathological reaction patterns, both in the epidermis and dermis.
Keywords
Dermoscopy
pathology
reaction
tattoo
Plain Language Summary
Tattoo-associated complications are increasing due to the popularity of decorative tattoos in India. The exact cause of various tattoo-associated reactions is not well-known and their dermoscopic features are rarely reported. This study aimed to delineate patients' clinical, dermoscopic and immunopathological details with tattoo reactions that were not infectious or eczematous. The authors evaluated the aforementioned features of noninfectious noneczematous tattoo reactions in five patients with six lesions. Morphologically, the reactions presented as erythematous or blue-gray flat-topped to verrucous papules and plaques. Dermoscopy showed a central white to pink-white structureless area, a peripheral gray-white to bluish-white structureless area, white scales, comedo-like opening with keratotic plugging, milia-like cysts and shiny white structures. All but one lesion had a combination of pathological reaction patterns. Immunohistochemistry showed increased epidermal and dermal Langerhans cells, predominantly CD8 positive T cells in the epidermis and dermis, sparse dermal B cells and CD4 positive T cells, reduced T regulatory cells and a complete absence of CD56 positive natural killer cells. The authors concluded that clinical morphology and dermoscopy might not differentiate between various types of noninfectious noneczematous inflammatory tattoo reactions and CD8 T cells play a central role in executing various pathological reaction patterns, both in the epidermis and dermis.
Introduction
The popularity of decorative tattoos in recent years has led to an increase in tattoo-associated complications. The complications can be broadly divided into infectious, inflammatory, and neoplastic groups.1,2 The inflammatory group encompasses a spectrum of reaction patterns, such as eczematous, lichenoid, granulomatous, pseudoepitheliomatous hyperplasia and pseudolymphoma and their diagnosis solely depend upon histopathological examination.3 The dermoscopic details of various inflammatory tattoo reaction patterns are sparsely reported. In addition, the exact pathogeneses of various reaction patterns are still not clear. We aimed to retrospectively study the clinical, dermoscopic and immunopathological details of patients with non-infectious non-eczematous inflammatory tattoo reaction patterns in a tertiary care centre of East India.
Methods
This retrospective study was conducted in a tertiary care hospital after approval from the institute ethics committee. All the patients who had tattoo reactions between December 2018 to December 2021 were assessed for inclusion. All the pathologically-proven non-infectious non-eczematous inflammatory tattoo reactions were included in the study. Figure 1 shows details of the study methodology.
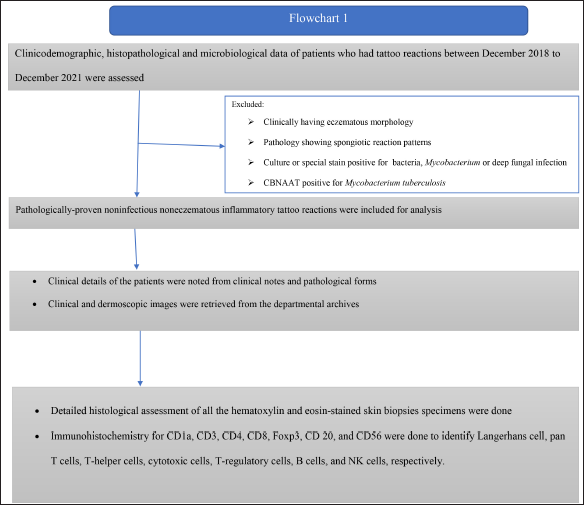
- Flowchart describing the study methodology
Clinical details of the patients were noted from clinical notes and pathology forms. All the clinical and dermoscopic images were retrieved from the departmental archives. Dermoscopic images were taken using DermLite, DL4 (Polarised mode, 10X magnification) dermatoscope attached to a Canon digital camera.
A detailed histological assessment of all the haematoxylin and eosin-stained skin biopsies specimens were done.
Immunohistochemistry for CD1a, CD3, CD4, CD8, FoxP3, CD 20 and CD56 were done to identify Langerhans cell, pan T cells, T-helper cells, cytotoxic cells, T-regulatory cells, B cells, and NK cells, respectively. Table 5 delineates the technical details of all the immunostains. The location of the immunopositive cells were marked (epidermal, dermal and subcutis), and the degree of infiltration was interpreted as a proportion (For CD3 and CD20- out of the total number of lymphocytes, for CD4, CD8, and foxp3- out of the total number of CD3 positive cells). CD1a positive cells were interpreted as mild, moderate or marked increase in Langerhans cells in the epidermis and dermis.
Results
A total of five patients with six lesions satisfied the inclusion and exclusion criteria, and belonged to the skin phototypes IV and V. The time from the tattooing to the onset of the reaction varied from four to ten months. Of the six lesions, five had reactions at the site of a black tattoo [Figure 2a], and one reacted to the red tattoo [Figure 3a]. Clinically [Table 1], the lesions presented with erythematous or blue-grey flat-topped to verrucous papules and plaques. The main complaint was cosmetic disfigurement followed by itching.

- Verrucous plaques (arrow points to the biopsied plaque)
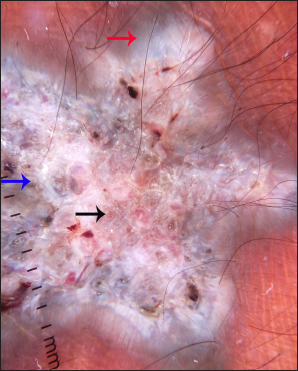
- Dermoscopy (DermLite DL4, ×10) under polarised mode shows central white structureless area (blue arrow) and peripheral grey-white structureless area (red arrow) along with comedo-like opening and keratotic plugging (black arrow)
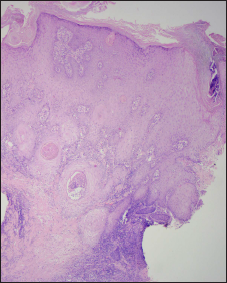
- Histology shows pseudoepitheliomatous hyperplasia (H & E, ×50)
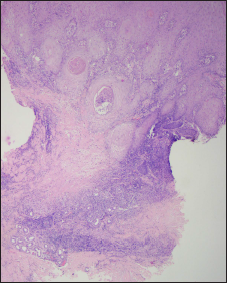
- Necrobiotic reaction pattern (H & E, ×50)
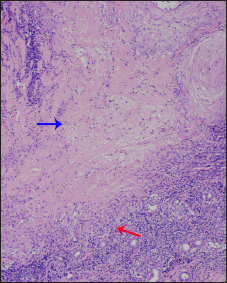
- Central area of necrobiosis (blue arrow) surrounded by palisaded histiocytic granulomas (red arrow) along with lymphocytes. Note the nuclear dust in the area of necrobiosis (H & E, ×100)
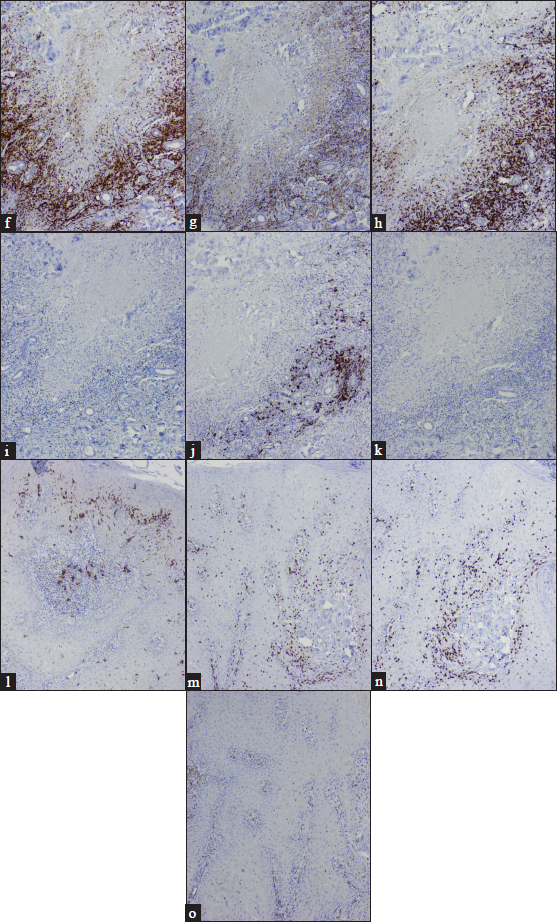
- (f) Dermal CD3 positive T lymphocytes (IHC, ×100); (g) Few CD4 positive T lymphocytes (IHC, ×100); (h) Majority of the cells are CD8 positive T lymphocytes (IHC, ×100); (i) Reduced Foxp3 positive T lymphocytes (IHC, ×100); (j) Occasional CD20 positive B lymphocytes, (IHC, ×100); (k) Negative CD56 positive natural killer T cells (IHC, ×100); (l) Increased epidermal CD1a positive Langerhans cells (IHC, ×400); (m) Epidermal CD3 positive T lymphocytes (IHC, ×400); (n) CD8 positive T lymphocytes (IHC, ×400); (o) CD4 negative T lymphocytes (IHC, ×400)

- Erythematous flat-topped plaque along the distribution of the red tattoo and sparing of the blue-black tattoo
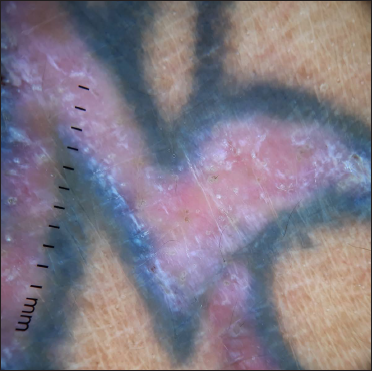
- Dermoscopy (DermLite DL4, ×10) under polarised mode shows pink-white structureless area, comedo-like opening and shiny white structures
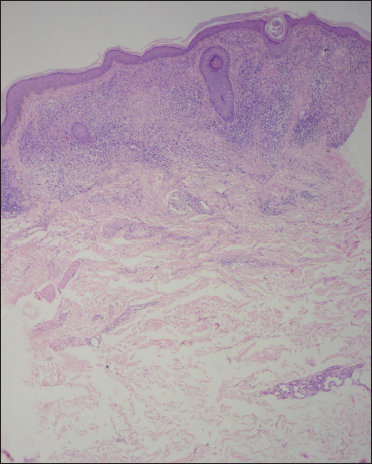
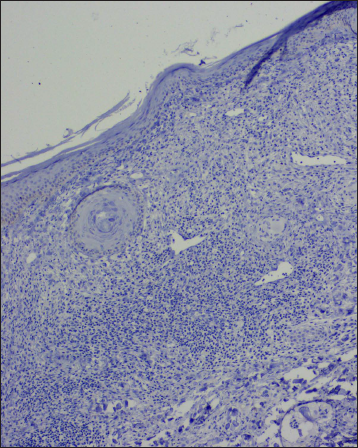
- Histology shows a lichenoid reaction pattern (H & E, ×50)
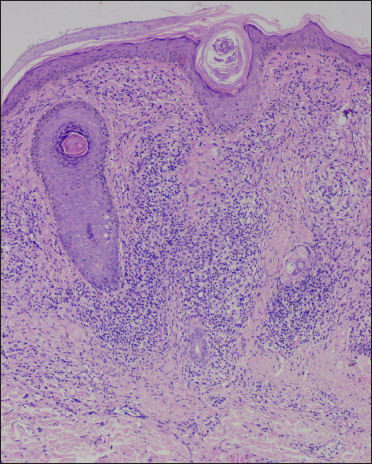
- Sub-epidermal band-like lymphohistiocytic infiltration with occasional giant cell (H & E, ×100)
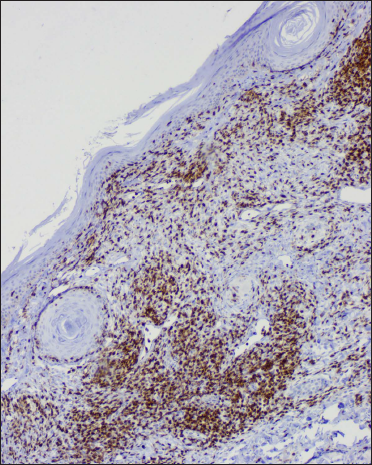
- Dermal CD8 positive T lymphocytes (IHC, ×100)
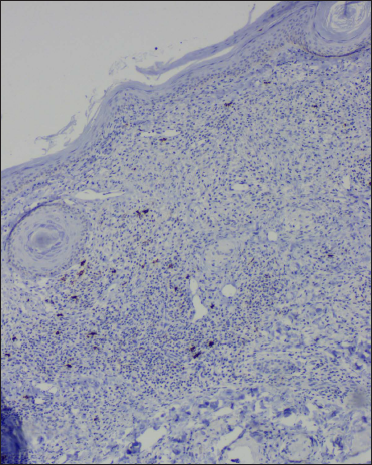
- Occasional CD20 positive B lymphocytes (IHC, ×100)
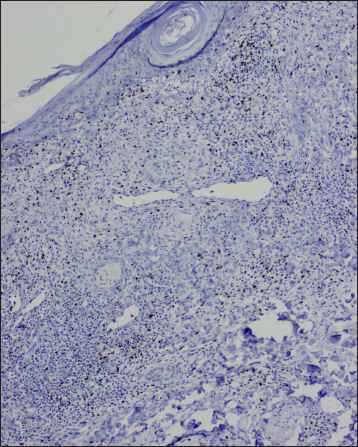
- Reduced Foxp3 positive T lymphocytes, (IHC, ×100)
- Negative CD56 positive natural killer T cells (IHC, ×100)
| Age/gender | Clinical morphology(tattoo colour) | Dermoscopic features | |
|---|---|---|---|
| Case 1 | 23/M | Erythematous to blue-grey verrucous plaque (A) (Black) |
Diffuse white to yellowish-white scales Central pinkish-white to bluish-white structureless area Peripheral blue-grey structureless area Comedo-like opening with keratotic plugging Shiny white lines, clods and structureless area Focal multicoloured pattern Focal hairpin and linear vessels |
| Blue-grey flat-topped papules (B) (Black) |
Focal white scales Bluish-white structureless area Shiny-white lines, clods and structureless area Focal hairpin vessels |
||
| Case 2 | 16/M | Erythematous to blue-grey flat-topped to verrucous papules and plaques (Black) |
Focal white scales Whitish to pinkish-white structureless area Focal grey-white to bluish-white structureless area Comedo-like opening with keratotic plugging Milia-like cysts Shiny white lines Focal linear and hairpin vessels |
| Case 3 | 40/M | Erythematous flat-topped plaque along the distribution of the red tattoo colour (Red) |
Diffuse white scales Pink-white structureless area Comedo-like opening keratotic plugging Shiny white lines, rosette, and clod Focal linear, and linear irregular vessels |
| Case 4 | 30/M | Blue-grey flat-topped to verrucous papules and plaque (Black) |
Focal white scales Pinkish-white, grey-white to bluish-white structureless area Comedo-like opening with keratotic plugging Shiny white lines and rosette |
| Case 5 | 26/M | Erythematous verrucous plaque (Black) |
Diffuse white scales Central pinkish-white structureless area Peripheral grey-white structureless area Comedo-like opening and follicular plugging Milia-like cysts Shiny white lines, clods, and rosette Grouped dotted vessels |
Dermoscopic features [Table 1] were dominated by a central white to pink-white structureless area and a peripheral grey-white to bluish-white structureless area. In addition, white scales, comedo-like opening with keratotic plugging, milia-like cysts and shiny white structures were noted in all the cases [Figures 2b and 3b]. Vascular structures mainly were focally distributed and included hairpin and linear vessels.
Pathologically [Figures 2c-e and 3c-d], except for one lesion that only showed a lichenoid reaction pattern in the red tattoo, all had a combination of reaction patterns that included lichenoid, pseudoepitheliomatous, interstitial and tuberculoid, sarcoid and necrobiotic granulomatous reactions [Table 2].
| Pathological features | Case 1 A | Case 1B | Case 2 | Case 3 | Case 4 | Case 5 |
|---|---|---|---|---|---|---|
| Reaction pattern | PEH + necrobiotic granulomatous reaction pattern | Sarcoidal + necrobiotic granulomatous reaction pattern | Necrobiotic + tuberculoid granulomatous reaction pattern | Lichenoid reaction pattern | Lichenoid + tuberculoid granulomatous reaction pattern | Lichenoid + interstitial granulomatous reaction pattern |
| Compact hyperkeratosis | + | + | + | + | + | + |
| Parakeratosis | + | + | + | + | + | − |
| Melanin in stratum corneum | + | + | + | + | + | + |
| Follicular dilatation and plugging | + | − | + | + | + | + |
| Hypergranulosis | − | − | + | − | + | + |
| Acanthosis (mild/moderate/marked) | Marked with PEH | Moderate | Moderate | Atrophy | Moderate | Moderate |
| Spongiosis (mild/moderate/severe) | − | Mild | − | − | − | − |
| Exocytosis (Mild/moderate/severe) |
L Mild |
L Mild |
L Moderate |
L Mild |
L Mild |
L Moderate to severe |
| Size of intraepidermal lymphocytes (small/medium/large) | Small | Small | Small to medium | Small | Small | Small to Medium |
| Basal vacuolar degeneration | + Focal |
+ Focal |
− | + | + | + |
| Cytoid bodies | + Focal |
− | − | + | + | + |
| Pigment incontinence | + | + | + | + | + | + |
| Grenz zone | − | − | − | − | − | − |
| Inflammatory infiltration (mild/moderate/marked) Inflammatory cells (+/++/+++) |
Marked L+++/H+++/P+/E+ |
Marked L++/H+++/P-/E- |
Marked L++/H++/P+/ E+ |
Marked L+++/H+/E+ |
Marked L+++/H+++/P-/ E- |
Marked L++/H++/P-/E+/ M+/- |
| Subepidermal lichenoid infiltration | − | − | − | + | + | − |
| Perivascular infiltrate | + | + | + | + | + | + |
| Perieccrine infiltrate | + | _ | + | − | + | + |
| Perineural infiltrate | − | − | − | − | − | + |
| Perifollicular infiltrate | + | − | + | + | + | − |
| Interstitial infiltrates | + | − | − | − | − | + |
| Granuloma (Tuberculoid/sarcoid/necrobiotic) | Necrobiotic | Necrobiotic and Sarcoidal | Necrobiotic and tuberculoid | − | Tuberculoid | Interstitial |
| Blood vessels | Dilated | Dilated | Dilated | Dilated | Dilated | Dilated |
| Fibrosis | − | + | + Perifollicular |
+ | + | + |
| Dilated lymphatics | − | − | − | − | − | − |
| Subcutaneous inflammation | Lobular lymphocytic infiltration | − | − | − | + | + |
| Depth of inflammation (up to papillary/upper reticular/lower reticular) | Subcutaneous tissue | Upper reticular dermis | Upper reticular dermis | Upper reticular dermis | Subcutis | Subcutis |
| Depth of tattoo pigment (papillary/upper reticular/lower reticular) | − | Upper reticular dermis | − | Upper reticular dermis | Lower reticular dermis | − |
Present (+), Absent (-), PEH: Pseudoepitheliomatous hyperplasia, L: Lymphocyte, H: Histiocyte, E: Eosinophil, P: Plasma cell, M: Mast cell
CD1a highlighted increased epidermal and dermal Langerhans cells. The dermal lymphocytes were predominantly of T cell lineage, with only a few cells stained for B cells. CD4 positive T cells constituted only a minor subset, and CD8 positive T cells were the dominant cell type of the dermal T-cells. In addition, there was a reduction in Foxp3 positive T regulatory cells and a complete absence of CD56 positive NK cells [Table 3]. A similar pattern of immunostaining for lymphocytes was noticed for the epidermal lymphocytes [Figures 2f-o and 3e-h].
| IHC | Case 1A | Case 1B | Case 2 | Case 3 | Case 4 | Case 5 |
|---|---|---|---|---|---|---|
| CD1a (Increase -mild/moderate/marked) |
Epidermis- Moderate Dermis- Mild |
Epidermis- Moderate Dermis- Mild |
Epidermis- Moderate Dermis-Moderate |
Epidermis- Mild to Moderate Dermis- Mild |
Epidermis- Moderate Dermis- Moderate |
Epidermis- Marked Dermis- Mild |
| CD3 | 95-99% | 95-99% | 90-95% | 90-95% | 95-99% | 85-90% |
| CD4 | 20-25% | 15-20% | 1-5% | 35-40% | 20-25% | 20-25% |
| CD8 | 70-75% | 85-90% | 90-95% | 60-65% | 70-75% | 75-80% |
| CD20 | 1-5% | 1-5% | 1-5% | 1-2% | 1-5% | 10-15% |
| FoxP3 | 5-9% | 5-10% | 5-10% | 10-15% | 1-5% | 5-10% |
| CD56 | Negative | Negative | Negative | Negative | Negative | Negative |
Discussion
Inflammatory tattoo reaction patterns can have heterogeneous non-specific clinical manifestations, and it is not easy to differentiate between them solely based upon the clinical morphology, as demonstrated in this study.4 The only exception was the lichenoid reaction pattern that presented with erythematous flat-topped plaque over the red tattoo without a verrucous surface, as reported before.2
There are only a few case reports on dermoscopic features of tattoo reactions. In the present series, the common dermoscopic features observed, irrespective of their tattoo reaction patterns, were a central pink-white structureless area, a peripheral grey-white to bluish-white structureless area, white scales, comedo-like opening with keratotic plugging, and shiny white structures. Shiny white structures were rosette, shiny white lines, clods, and structureless areas. The central pink-white structureless area corresponds to the acanthotic epidermis with increased vascularity, and the peripheral bluish-white to grey-white structureless area to the acanthotic epidermis and dermal black tattoo pigment. The blue-white to grey-white structureless area and Wickham’s striae were absent in the lichenoid tattoo reaction.
In contrast to the previously reported orange to yellowish-orange structureless area in granulomatous tattoo reaction and sarcoid foreign body reaction,5 we observed a white to pinkish-white structureless area; This may be due to the overlying epidermal hyperplasia masking the mass effect of the dermal granuloma or may be due to lack of compact or conglomerate granulomas needed to produce the mass effect (yellow colour). A prior case of tattoo pseudolymphoma demonstrated a homogenous violaceous pattern with a follicular white-yellow halo.6 Pohl et al. advocated for dermoscopic examination of all the tattoos before laser removal to look for any pigmented lesions and, if present, tattoos not to be treated with laser.7
The histopathological spectrum of non-infectious non-eczematous tattoo reaction patterns can be broad and include the following: lichenoid, psoriasiform, pseudoepitheliomatous hyperplasia, granulomatous, keratoacanthoma-like, cutaneous lupus erythematosus-like and pseudolymphomatous.8-10 In this series, all but one tattoo reaction had more than one type of pathological reaction patterns. Lymphocytes and histiocytes were the dominant inflammatory cells in the dermis, while epidermal exocytosis demonstrated only lymphocytes. None of them showed eosinophilic or plasma cell infiltration except for one lesion. A recent study reported a combination of interface dermatitis and histiocytic hypersensitivity reactions in red tattoo-associated reactions.9 In our study, a similar pattern was observed, but all secondary to black tattoos.
Non-infectious granulomatous tattoo reaction patterns can be tuberculoid, sarcoidal and necrobiotic. It is mandatory to do a culture and, or special stain for acid-fast bacilli to rule out tuberculosis and leprosy, two major public health problems in India. Sarcoidal granulomatous pattern needs special mention, as it can be a part of systemic sarcoidosis or simple sarcoidal type foreign body granulomatous tattoo reaction pattern. The clinical morphology, even the dermoscopy, may not be able to distinguish between the two. So, it is binding to rule out systemic involvement.2,11,12 The only case in this series did not have any features of systemic sarcoidosis. Necrobiotic tattoo granuloma is an exceptionally rare type of tattoo reaction pattern. Both granuloma annulare-like and necrobiosis lipoidica-like necrobiotic tattoo reaction patterns have been described.13,14 All three cases in this study showed palisading necrobiotic granulomas.13 Table 4 depicts the clinical morphology and associated reaction patterns linked to different tattoo colours.15
| Colour of tattoo | Cutaneous manifestation | Histopathological pattern |
|---|---|---|
| Black | ● Hyperpigmented plaque with or without erosions ● Lichenoid plaque ● Discrete hyperpigmented papule ● Eczematized plaque ● Erythematous plaque ● Crusted lesion over an erythematous base |
● Allergic ● Lichenoid ● Granulomatous |
| Red | ● Erythematous plaque ● Hyperpigmented plaque with or without crusting |
● Allergic ● Lichenoid |
| Green | ● Hyperpigmented plaque with or without erosions ● Pruritic eczematized plaque |
● Allergic ● Granulomatous |
| Immunostains | Sites of positivity | Highlighted cells | Dilution | Clone | Company |
|---|---|---|---|---|---|
| CD1a | Membranous | Langerhans cell | Ready-to-use | − | Biocare, California, USA |
| CD3 | Membranous | Pan T cell | 1:100 | Rabbit polyclonal |
PathnSitu, Livermore, CA, USA |
| CD4 | Membranous | T-helper cell | Ready-to-use | 4B12 | Dako, Carpinteria, CA, USA |
| CD8 | Membranous | Cytotoxic T cell | Ready-to-use | C8/144B | Dako, Carpinteria, CA, USA |
| FoxP3 | Nuclear | T-regulatory cell | 1:400 | 236A/E7 | Abcam, Cambridge, MA, USA |
| CD20 | Membranous | B cell | 1:100 | L26 | PathnSitu, Livermore, CA, USA |
| CD56 | Membranous | NK cells | Ready-to-use | 123C3 | Dako, Carpinteria, CA, USA |
The use of the patch test in tattoo reactions is not helpful. A study on patch test in patients with tattoo reactions showed that patients with clinically significant reactions in their tattoos presented mainly negative or inconsistent results when patch tested with common allergens, textile dyes, problematic tattoo ink stock products and even with their individual culprit inks when available for testing.16
The exact aetiopathology of different types of non-infectious non-eczematous inflammatory tattoo reaction patterns are unknown. A tattoo ink-associated hypersensitivity reaction has been postulated to cause these delayed types of reaction patterns.6
In our study, the immunohistochemistry revealed a cytological profile similar to a delayed-type of hypersensitivity in all the cases, irrespective of the tattoo’s colour or pathological reaction patterns. In all the cases, T cells were the predominant lymphocytes, and B cells were sparse. The epidermal T lymphocytes were mostly CD8 positive T cells and responsible for the interface dermatitis and epidermal reaction. The dermal T lymphocytes were predominantly CD8 positive T cells and acted as the effector cell in orchestrating the delayed types of reaction patterns. In addition, increased epidermal and dermal Langerhans cells, and dermal sparse CD4 positive cells, and Foxp3 T-regulatory cells, as demonstrated, support the same concept. As described before, CD20 positive B-cells and CD56 positive NK T-cells do not appear to have any significant role in the tattoo reactions.4
The limitations of our study were the small sample size and the absence of other variants of inflammatory tattoo reaction patterns.
In conclusion, the clinical morphology and dermoscopy did not help differentiate between various types of reaction patterns. A combination of pathological reaction patterns is commonly observed in histopathology. The immunological profile supports a delayed hypersensitivity reaction due to contact sensitisation to tattoo pigment, and CD8 T cells play a central role in executing various pathological reaction patterns. Future studies with large sample sizes and comparison groups may shed light on the role of dermoscopy in detecting and differentiating between various non-infectious tattoo reactions.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflict of interest
There are no conflicts of interest.
References
- Tattoo complaints and complications: Diagnosis and clinical spectrum. Curr Probl Dermatol. 2015;48:48-60.
- [CrossRef] [PubMed] [Google Scholar]
- Medical complications of tattoos: A comprehensive review. Clin Rev Allergy Immunol. 2016;50:273-86.
- [CrossRef] [PubMed] [Google Scholar]
- Tattoo-associated skin reaction: The importance of an early diagnosis and proper treatment. Biomed Res Int. 2014;2014:354608.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Histopathology and immune histochemistry of red tattoo reactions. Interface dermatitis is the lead pathology, with increase in T-lymphocytes and Langerhans cells suggesting an allergic pathomechanism. Skin Res Technol. 2015;21:449-58.
- [CrossRef] [PubMed] [Google Scholar]
- Orange color: A dermoscopic clue for the diagnosis of granulomatous skin diseases. J Am Acad Dermatol. 2015;72:S60-3.
- [CrossRef] [PubMed] [Google Scholar]
- Dermoscopy of a tattoo pseudolymphoma. Dermatol Pract Concept. 2019;9:17-9.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Pitfalls and recommendations in cases of laser removal of decorative tattoos with pigmented lesions: Case report and review of the literature. JAMA Dermatol. 2013;149:1087-9.
- [CrossRef] [PubMed] [Google Scholar]
- The histopathologic spectrum of decorative tattoo complications. J Cutan Pathol. 2012;39:1110-8.
- [CrossRef] [PubMed] [Google Scholar]
- Histopathology of red tattoo reactions. Am J Dermatopathol. 2021;43:331-7.
- [CrossRef] [PubMed] [Google Scholar]
- Cutaneous lymphoid hyperplasia (pseudolymphoma) in tattoos: A case series of seven patients. J Eur Acad Dermatol Venereol. 2010;24:208-13.
- [CrossRef] [PubMed] [Google Scholar]
- Necrobiotic granulomatous tattoo reaction: Report of an unusual case showing features of both necrobiosis lipoidica and granuloma annulare patterns. Am J Dermatopathol. 2014;36:e152-5.
- [CrossRef] [PubMed] [Google Scholar]
- A novel inflammatory reaction in a tattoo: Challenge. Am J Dermatopathol. 2011;33:740-9.
- [CrossRef] [PubMed] [Google Scholar]
- Histopathological evaluation of cutaneous reactions to tattoos: Study at a tertiary care center. J Cutan Pathol. 2021;48:870-6.
- [CrossRef] [PubMed] [Google Scholar]
- Patch test study of 90 patients with tattoo reactions: Negative outcome of allergy patch test to baseline batteries and culprit inks suggests allergen(s) are generated in the skin through haptenization. Contact Dermatitis. 2014;71:255-63.
- [CrossRef] [PubMed] [Google Scholar]






