Translate this page into:
Clinicopathological correlation of acquired hyperpigmentary disorders
2 Delhi Dermatology Group, Delhi Dermpath Laboratory, New Delhi, India
Correspondence Address:
Asha Kubba
10, Aradhana Enclave, R.K. Puram, Sector 13, New Delhi - 110 066
India
| How to cite this article: Patel AB, Kubba R, Kubba A. Clinicopathological correlation of acquired hyperpigmentary disorders. Indian J Dermatol Venereol Leprol 2013;79:367-375 |
Abstract
Acquired pigmentary disorders are group of heterogenous entities that share single, most significant, clinical feature, that is, dyspigmentation. Asians and Indians, in particular, are mostly affected. Although the classic morphologies and common treatment options of these conditions have been reviewed in the global dermatology literature, the value of histpathological evaluation has not been thoroughly explored. The importance of accurate diagnosis is emphasized here as the underlying diseases have varying etiologies that need to be addressed in order to effectively treat the dyspigmentation. In this review, we describe and discuss the utility of histology in the diagnostic work of hyperpigmentary disorders, and how, in many cases, it can lead to targeted and more effective therapy. We focus on the most common acquired pigmentary disorders seen in Indian patients as well as a few uncommon diseases with distinctive histological traits. Facial melanoses, including mimickers of melasma, are thoroughly explored. These diseases include lichen planus pigmentosus, discoid lupus erythematosus, drug-induced melanoses, hyperpigmentation due to exogenous substances, acanthosis nigricans, and macular amyloidosis.Introduction
Acquired pigmentary disorders are found all over the world, but can be particularly distressing in patients with skin of color. Classic morphologies of these conditions have been reviewed in the global dermatology literature with an emphasis on treating these difficult diseases. [1] The importance of accurate diagnosis is emphasized here as the underlying diseases have varying etiologies that need to be addressed in order to effectively treat the dyspigmentation. In this review, we describe and discuss the utility of histology in the diagnostic work of hyperpigmentary disorders and how, in many cases, it can lead to targeted and more effective therapy. We focus on the most common acquired hyperpigmentary disorders seen in Indian patients as well as a few uncommon diseases with distinctive histological traits.
Facial Melanoses
This group of diseases has been the focus of many review and management-focused articles, both in Indian dermatology literature and abroad. Pigmentary alterations of the face can be extremely distressing to the patient, making their effective management a priority for both the patient and the physician.
Melasma
Melasma (chloasma) is the most common clinical diagnosis made among the facial melanoses. It occurs mostly in women, and is clinically characterized by patterned, symmetric, brown to grayish-brown, hyperpigmented patches of the face in malar and centro-facial areas [1] [Figure - 1]a. However, in India, in one reported series, one out of every five patients was male. [2]
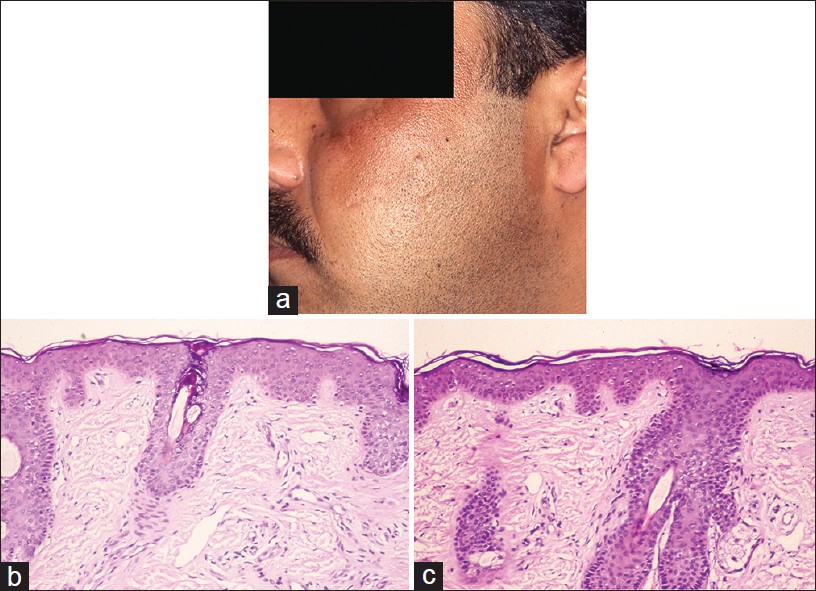 |
| Figure 1: Melasma: (a) Progressive darkening of face; asymptomatic, (b) Perilesional normal skin, pigmented (H and E, ×100), (c) Lesional: Epidermal hyperpigmentation. No inflammation, no dermal pigment (H and E, ×100) |
Hyperpigmentation is largely epidermal, causing accentuation of the lesions under Wood′s lamp. Melasma may, in some cases, present with significant dermal pigmentation, and this is clinically suggested by grayish hue and the lack of accentuation under Wood′s light examination. The clinical and histopathological correlation of increased dermal melanin and negative Wood′s lamp examination is controversial, Sanchez et al. [3] showing the correlation and Grimes [4] denying it.
The diagnosis of melasma is usually made on the basis of clinical features alone. However, this approach is unsatisfactory as there are many pigmentary disorders that, clinically, mimick melasma and the distinction between them invariably requires astute histopathological evaluation and subsequent clinical correlation. The histopathological features of melasma per se are subtle and only when control skin biopsies are available for comparison, they are truly appreciated [3],[4] [Figure - 1]b and c. Increased epidermal melanin is the key feature and this requires Masson Fontana stain. This increase is seen mainly in the basal/suprabasal cells as pigmentary caps [3],[4] but in a Korean study increased melanization was noted in all layers of the epidermis [5] Dermal melanin is observed in some cases (dermal melasma) mainly in macrophages (also called melanophages) but also as free melanin deposits around superficial and deep vascular plexuses. [3] Increased melanocytic activity has been documented in melasma using Mel-5 immuno-staining. [4],[5] It reportedly varies from actual increase in the number of melanocytes [5] to merely enlargement of melanocytes with increased staining and prominent dendrites indicative of increased melanogenesis. [4]
Melasma pathology also includes additional dermal changes, namely solar elastosis and telengiectasia. These changes are best appreciated when uninvolved skin is available for comparison. Solar elastosis is evaluated with Verhoeff-van Gieson stain (VVG) and it has been observed in melasma as clumps of thick and fragmented elastic fibers in the papillary dermis. Sarkar et al. [2] in their series, reported solar elastosis in 85% of 20 biopsies from melasma patients. The histopathological evaluation of melasma should be done to exclude melasma mimickers. The best practice is to compare the pigmentary alteration of lesional skin with patient′s normal skin as control.
Lichen Planus Pigmentosus
Lichen planus pigmentosus (LPP) is a macular variant of lichen planus reported mostly from the Indian subcontinent and the Middle-East. [6],[7] It is characterized by focal or coalescing gray-blue patches or subtle plaques arising on the exposed areas, [Figure - 2]a especially the neck and the adjacent torso. [1],[6] It is typically bilaterally symmetrical. [7] Rarely, LPP can mimick melasma. Photosensitivity is reported by some patients. The hyperpigmentation is mainly dermal, resulting in lesions that do not accentuate under Wood′s lamp. Ashy dermatosis/erythema dyschromicum perstans bears similar morphology and is pathologically indistinguishable except it has predilection for non-sun exposed areas and has been reported mostly from South America. [8] LPP inversus is yet another similar entity in which intertriginous areas are predominantly involved. [9]
 |
| Figure 2: Lichen planus pigmentosus (a) Macular hyperpigmentation, face, (b) Basal cell damage, necrotic keratinocyte and pigment incontinence (H and E, ×100) |
LPP being a subset of lichen planus may, on occasion, exhibit typical lichenoid pathology if palpable lesions are present and are sampled. In most instances, however, that is not the case. Histopathological evaluation of a macular lesion shows a relatively flat epidermis with loss of rete pattern, focal basal cell vacuolization with occasional necrotic keratinocyte and Civatte body, dermal melanophages, and a sparse lymphohistiocytic inflammation [Figure - 2]b. Foci of squamatized epidermis are sometimes encountered and indicate preceding vacuolar damage of the basal layer in a relatively aged lesion.
In a study, [7] in which 65 skin biopsies of LPP were examined, basal cell vacuolization was documented in 78.5%, dermal melanophages in 63%, and perivascular infiltrate in 81.5% of the cases. In the same study, band-like lichenoid infiltrate was observed in only 18.5% of the cases. The histopathology of LPP is, in general, subtle with sparse inflammation, requiring thorough evaluation of the biopsy. Therefore, diagnosis of LPP is based on clinicopathological correlation.
Discoid Lupus Erythematosus
Discoid lupus erythematosus (DLE) may present as hyperpigmented plaques on the face and neck in a focal distribution without attendant erythema, induration, adherent scaling or scarring. This presentation of DLE is rarely reported in the literature. [10],[11] In a clinical study of LE from India, pigmented macular form was recorded in 25% of 65 cases. [10] We illustrate this entity with a case of a 31-year-old female who over a 3-year period progressively developed hyperpigmented plaques on the forehead, temples, cheeks, ears, and eyelids associated with photosensitivity [Figure - 3]a. She was unsuccessfully treated for melasma. The diagnostic clues were bilateral asymmetry and follicular plugs. Wood′s lamp examination showed accentuation. Histopathology showed a relatively thin epidermis with focal basal cell vacuolization, necrotic keratinocytes, pigment incontinence along with superficial and deep perivascular and perifollicular infiltrate of lymphohistiocytes [Figure - 3]b and c. Follicular ostia were dilated and plugged. The clinical diagnosis of LE was validated using direct immunofluorescence study which showed a granular band of IgM and C3 along the basement membrane zone.
Melanotic LE as a melasma mimicker is hitherto unreported. Melanotic LE as a subset of DLE is, in our opinion, more common than the paucity of published literature would suggest. Both hypopigmentation and hyperpigmentation are recognized sequelae of LE. A good history and evidence of scarring would readily identify this group. A true melanotic LE would be when the hyperpigmentation arises de novo without preceding clinical signs of inflammation. Melanotic LE is more likely to be confused with LPP. However, with histopathology and DIF, it is not difficult to distinguish between them.
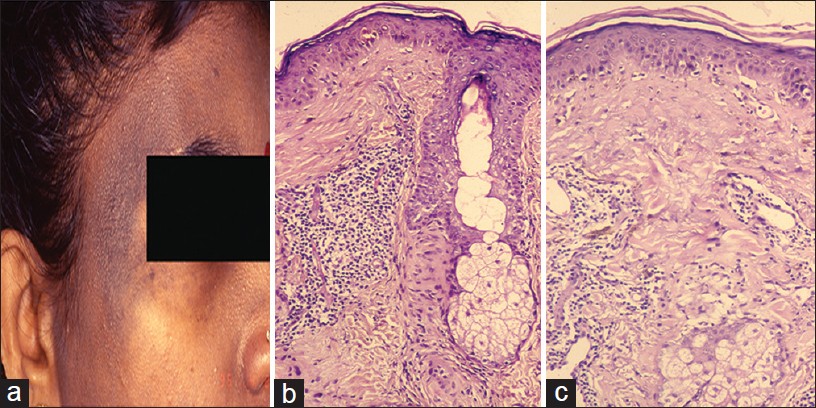 |
| Figure 3: Melanotic LE (a) Macular pigmentation, temple with follicular plugging, (b) Basal cell vacuolization, necrotic keratinocyte, perifollicular inflammation (H and E, ×100), (c) Necrotic keratinocyte, dermal mucin and inflammation (H and E, ×100) |
Drug-Induced Melanosis
Pigmentation as a side effect of medications is well recognized. Clofazimine, minocycline, and antimalarial-induced hyperpigmentation show varying pathologies but all are time-sensitive to the onset of the medication.
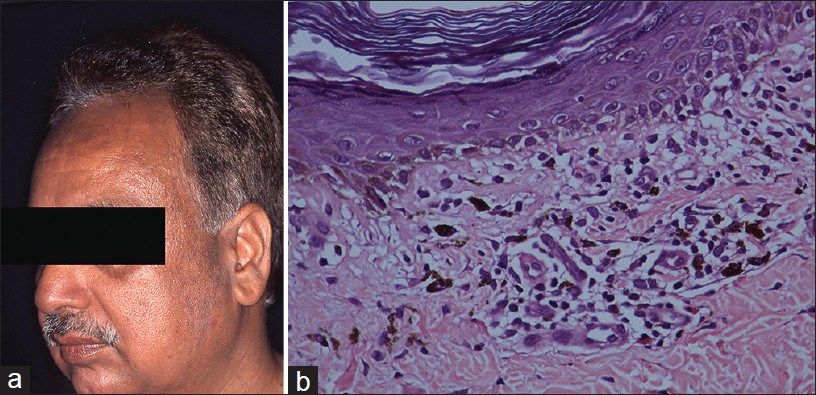
Most notable is clofazimine-induced brown pigmentation that is accentuated in sun-exposed areas and at times may appear like melasma , [Figure - 4]a but typically it is generalized and nails are commonly involved [Figure - 4]b. Two types of pigments accumulate in the skin, namely, redox dye and lipofuscin-ceroid. Redox dye is deposited in the skin, fat, gut, and lymph nodes usually within weeks of starting therapy. Discoloration of urine and sweat may also occur. Lipofuscin-ceroid pigment appears later, typically after 6-12 months, and is reversible. [12]
 |
| Figure 4: Clofazimine pigmentation (a) Facial melanosis, (b) Pigmentation of nails, (c) Pigmented macrophages in deep dermis (H and E, ×400) |
Histopathology of clofazimine-induced pigmentation is characterized by large collections of foamy macrophages in the dermis bearing brownish granular pigment [Figure - 4]c. The two pigments are indistinguishable and do not stain with acid fast stain, melanin stain, or iron stain. Under ultraviolet light, the pigments appear golden brown. Redox dye is best visualized in frozen sections as red auto-fluorescent deposits and it appears as birefringent crystals by light microscopy. [13] Lipofuscin-ceroid is identified by Mallory′s hemofuscin stain in paraffin-embedded sections. Epidermis shows loss of rete pattern.
Minocycline-induced pigmentation is seen as perivascular granular brown pigment that stains positive with Perl′s and Masson Fontana. Antimalarial-induced yellow-brown pigment is distributed within histiocytes throughout the dermis. It is weakly positive with Perl′s stain.
Argyria
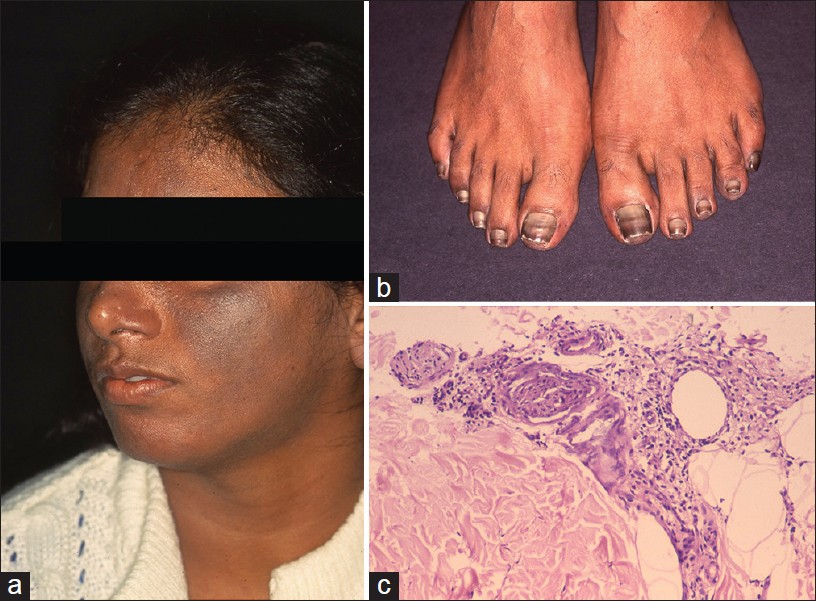
Argyria is an exotic cause of hyperpigmentation. It results from deposition of silver in the skin subsequent to prolonged ingestion of silver. [14],[15],[16] In India, the exposure is mainly through ingestion of silver-coated sweets (mithai with silver virq) and silver-coated cardamom, beetle nut, and fennel seeds (consumed as mouth freshners for years on end). Argyria produces distinctive gray-blue discoloration in a photo pattern. Similar discoloration can also be seen over ear cartilage, in the nails, mucous membranes, and sclera [1],[15] [Figure - 5]a and b.
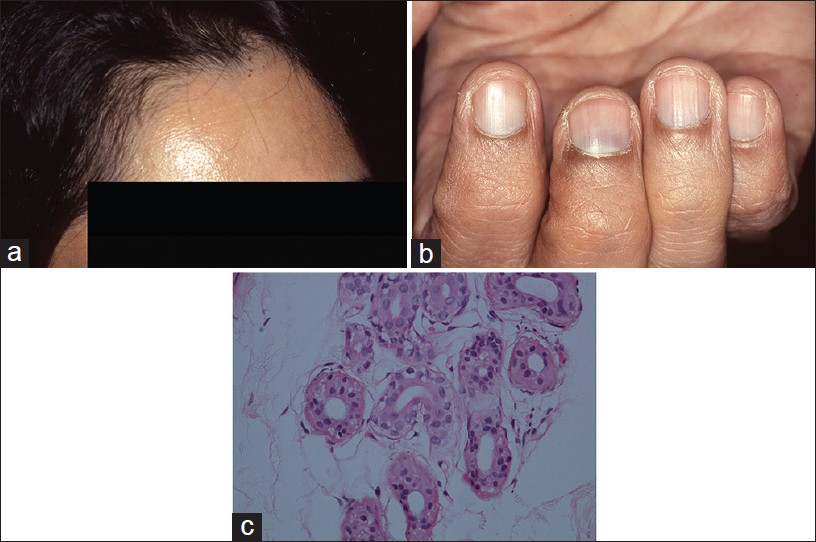 |
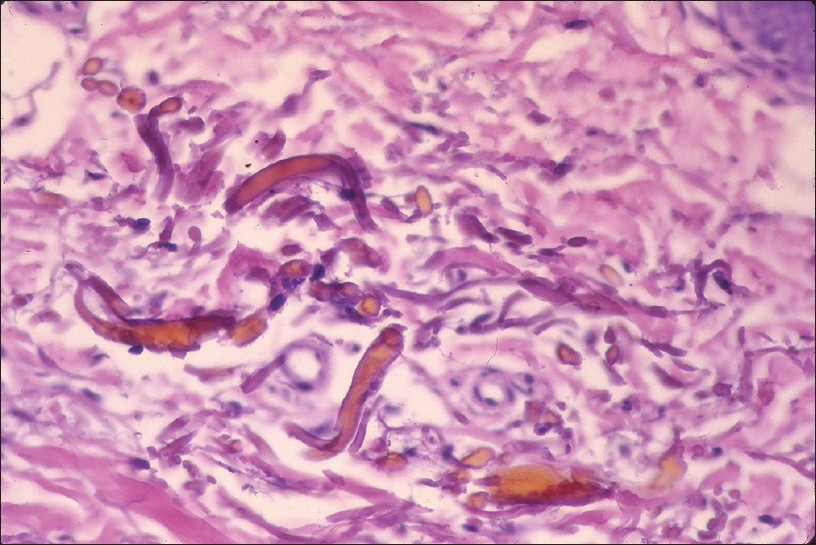
| Figure 5: Argyria (a) Blue-gray melanosis of face, (b) Blue discoloration of lunula of nails (c) Fine brown-black granules deposited around sweat gland basement membrane (H and E, ×100) |
Localized cutaneous argyria due to prolonged contact with silver salts or silver jewelry, or from penetration of acupuncture needles is also reported. Such lesions may mimick melanocytic lesions including melanoma. [17],[18] Argyria lesions do not accentuate on Wood′s lamp examination.
Histopathology shows brown-black granules deposited in a band-like fashion along the basement membrane of the eccrine glands [Figure - 5]c. Occasionally, similar granules may be seen in the papillary dermis corresponding to elastic fibers, and around pilosebaceous units. Additional sites to examine are blood vessel wall and arrector pili muscle. Granules are refractile under dark field microscopy. [15],[16] Argyria pathology is subtle and may present as an invisible dermatosis. There is no obvious evidence of pigmentary pathology such as interface damage or dermal melanophages. Silver being inert, inflammation is minimal or absent. Gold and mercury granules are also refractile. However, granules in chrysiasis are larger, irregular in shape, mostly intracellular within macrophages and endothelial cells, and show orange-red birefringence on fluorescent microscopy. Mercury granules are larger, irregular, and are found in upper dermis mostly within macrophages. Positive identification of silver in the tissue can only be done by atomic absorption spectrophotometry. [19],[20] In argyria, serum silver levels reportedly range from 4 to 40 g, whereas the total silver content in the body is 1 mg. [14] Diagnosis can be established by histopathology and positive history of silver intake.
Ochronosis
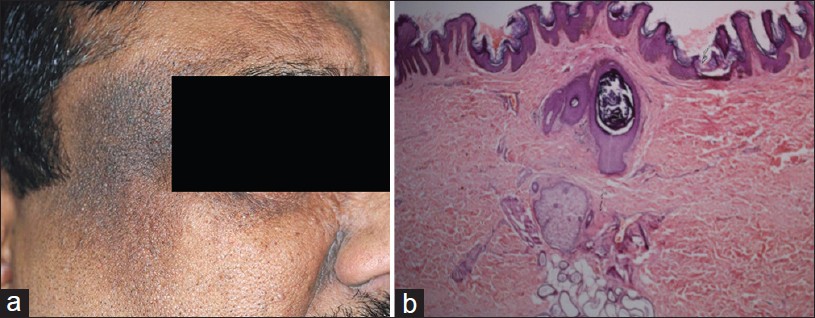
Exogenous ochronosis is a blue-black dermal discoloration secondary to prolonged use of skin bleaching creams containing hydroquinone. Bleaching occurs at first, followed by hyperpigmentation with papules over the treated areas. Clinically, pigmentation occurs over bony prominences, forehead, temples, nose, lower jaws, and sides of neck. [21] Exact pathogenesis of exogenous ochronosis is not known. It is believed that hydroquinone inhibits homogentisate 1, 2-dioxygenase in skin with formation of the pigment. Endogenous ochronosis (alkaptonuria) is an autosomal recessive disorder caused by inherited deficiency of homogentisic acid oxidase resulting in progressive deposition of polymerized homogentisic acid in the connective tissues of the skin and internal organs.
Histopathology of an early lesion shows swollen and basophilic collagen fibers. Older lesions show characteristic yellow-brown ochronotic pigment (homogentisic acid) coating collagen fibers and appearing as banana-shaped bodies [Figure - 6]. Pigment granules are often also present in epithelium and basement membrane of sweat glands. The pigment is auto-fluorescent and it does not stain with Masson Fontana or Perl′s stain. Ochronotic fibers do not stain with elastic stain. [21] Yellow collagen fibers eventually become amorphous eosinophilic material resembling colloid milium. Melanophages may be observed in upper dermis. [21] Histopathological features are common to both endogenous and exogenous ochronosis. [22]
 |
| Figure 6: Ochronosis: Banana-shaped collagen fibers covered by ochronotic pigment (homogentisic acid) (H and E, ×100) |
Acanthosis Nigricans
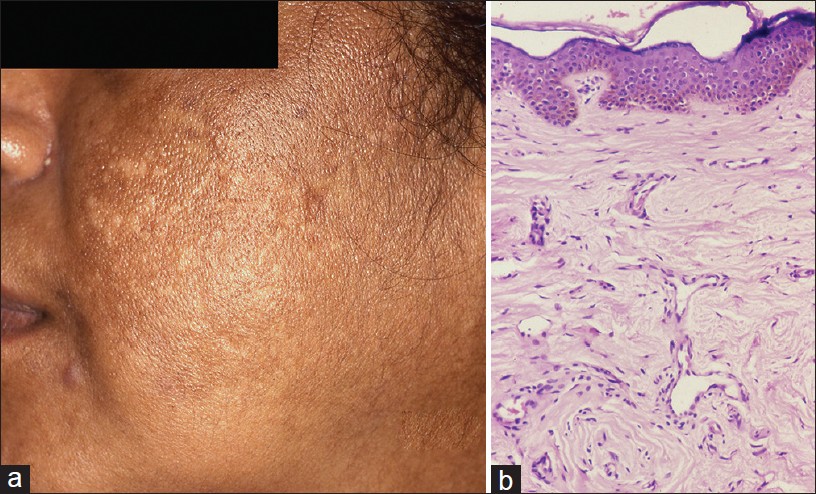
Acanthosis nigricans (AN) is characterized by hyperpigmented, velvety thickening of skin predominantly affecting nape of neck and intertriginous/flexural areas of the body. Acquired AN is associated with a variety of conditions especially insulin resistance, metabolic syndrome, and malignancies. Obesity associated with AN was reported in 70% of patients in an Indian study. [23] AN is also a feature of several genetic syndromes.
Clinically, AN lesions present as symmetrical velvety plaques with predilection for back of neck, axillae, and groins [Figure - 7]a. Less common sites include face, back of hands, fingers, or rarely palms. Oral lesions may involve tongue and lip. [23],[24]
 |
| Figure 7: Acanthosis nigricans (a) Macular hypermelanosis over temple. (b) Papillomatosis, acanthosis with pigmented basal epidermis (H and E, ×100) |
Histopathological features are subtle. Hyperkeratosis covers a papillomatous epidermis with finger-like projections forming peaks and valleys. Acanthosis is subtle [Figure - 7]b. Features seen less often are perivascular chronic inflammatory infiltrate in upper dermis and occasionally keratin cysts reminiscent of seborrhoeic keratosis. In a study of 30 patients of AN, dermal inflammation was seen in 60% and pseudocysts in 30% cases. [23] Clinically, hyperpigmentation does not reflect in the pathology which shows minimal increase in melanin. Pathology of AN is similar to epidermal naevi. The distinction is made on the basis of clinicopathological correlation.
Poikiloderma of Civatte
Poikiloderma of Civatte is most commonly seen in middle-aged, postmenopausal Caucasian women, but has also been reported in individuals with skin of color. [1] Skin lesions typically involve sun-exposed parts of face, neck, and V of the chest. Clinically, it presents as reticulate spots of hyperpigmentation and atrophic hypopigmentation with telengiectasia [25] [Figure - 8]a.
 |
| Figure 8: Poikiloderma of Civatte (a) Reticulated hyper- and hypopigmented atrophic macules, face, neck, (b) Atrophy, telengiectasia, and dermal fibrosis (H and E, ×100) |
Histology is characterized by telengiectasia, fibrosis, and melanophages in the papillary dermis with prominent solar elastosis [Figure - 8]b. Mild epidermal atrophy may be present particularly in older lesion. Accumulated elastotic material can be better visualized with an Orcein stain. Occasional mild vacuolar change is associated with melanin incontinence. [26] Poikiloderma associated with connective tissue disease shows interface dermatitis. Poikiloderma atrophicans vasculare displays band-like inflammation.
Riehl′s Melanosis (Pigmented Contact Dermatitis)
Riehl′s melanosis is thought to be a pigmented contact dermatitis to allergens present in cosmetics, fragrances, and kumkum. [27],[28] It presents as patches of diffuse gray-brown pigmentation on forehead, scalp, face, and neck [Figure - 9]a. Erythema, scaling, or pruritus is occasionally present. Henna application has been implicated in some patients. [1]
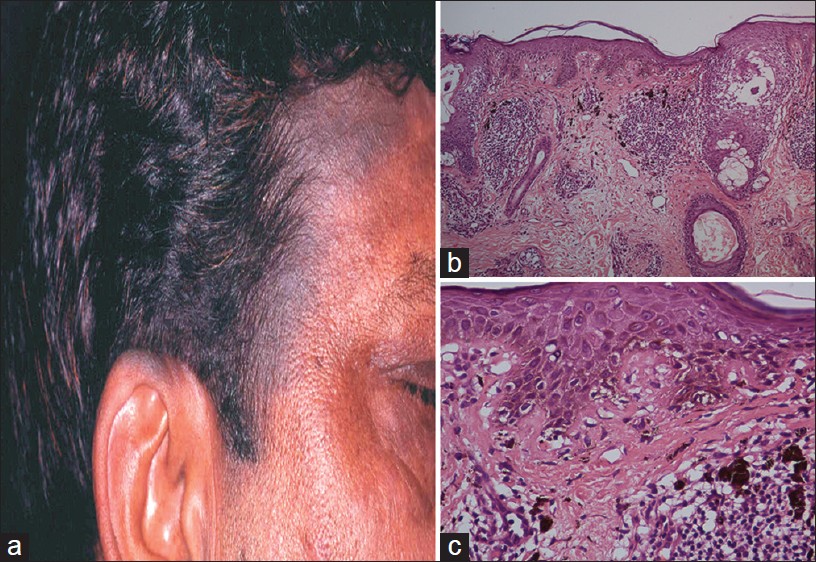 |
| Figure 9: Pigmented contact dermatitis (a) Diffuse brown pigmentation, face, forehead, ear. (b) Dermal inflammation, prominent pigment incontinence (H and E, ×100), (c) Basal cell damage, necrotic keratinocytes, Civatte bodies, melanophages, and dermal inflammation (H and E, ×400) |
Contrary to its name, histopathology, of pigmented contact dermatitis is not spongiotic but an, interface dermatitis with basal cell vacuolization [29] [Figure - 9]b and c. Superficial perivascular lymphocytic infiltrate and melanophages are present in the dermis. Interface dermatitis overlaps with lupus erythematosus (LE) and LPP. [1],[30] LE typically shows deep perivascular and perifollicular inflammation, and lupus band on direct immunofluorescence study. LPP, on the other hand, is difficult to distinguish from Riehl′s melanosis. The diagnosis is based on clinical correlation. Spongiosis and lymphocytic exocytosis are diagnostic features of contact dermatitis.
Hori′s Nevus (Acquired Bilateral Nevus of Ota Like Macules)
Hori′s nevus was first described in 1984 in Japanese women. [31] Unlike Nevus of Ota, it is an acquired condition that occurs late in life. It is an example of circumscribed melanocytosis, similar to nevus of Ota/Ito and Mongolian spot. It typically presents with large pigmented macule(s) on the face. Ocular and mucosal membranes are not involved. Clinical features most commonly are bilateral, on the malar cheeks, forehead, temples, and eyelids, as blue-brown or slate gray patches. Facial macules are similar to melasma and Riehl′s melanosis [Figure - 10]a. Pigmentation is not photosensitive. Skin lesions do not accentuate on Wood′s lamp examination as pigmentation is largely dermal. [31] Clinical similarity between melasma and Acquired bilateral nevus of Ota like macules (ABNOM) was further investigated for possibility of shared pathogenesis between the two entities. [32]
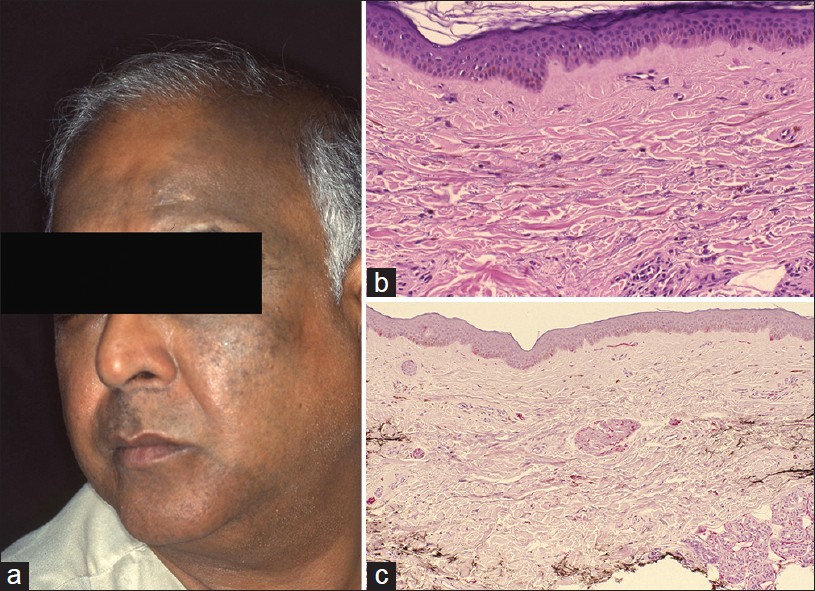 |
| Figure 10: Hori's nevus (a) Bilaterally, asymmetrical hyperpigmentation, forehead, temporal scalp, (b) Pigmented dermal melanocytes and melanophages in dermis (H and E, ×100), (c) Dermal melanocytes in the dermis are highlighted (S-100 stain, ×100) |
Histopathology is subtle on scanning power. A close scrutiny will show melanophages in the mid- and upper dermis [Figure - 10]b. Dermal melanocytes are hard to see as they are scattered diffusely in upper and mid-dermis, as single cells in between dermal collagen fibers. Elongated melanocytes may appear pigmented. The subtle pathology can be highlighted with Masson Fontana stain that highlights melanin pigment. [33] S-100 stain identifies the dermal melanocyte [Figure - 10]c. Melasma and Riehl′s melanosis do not show melanocytic pathology.
Macular amyloidosis
Macular amyloidosis is an example of cutaneous variant of amyloidoses, which is common in Middle-East and India. Women are affected more often than men. Macular dark brown, symmetrical pigmentation occurs on central, upper back, chest, extremities, and face. Characteristic lesions consist of rippled or reticulated pigmentation which is typically pruritic [Figure - 11]a. It is not photosensitive and Wood′s lamp examination does not accentuate the dermal pigmentation.
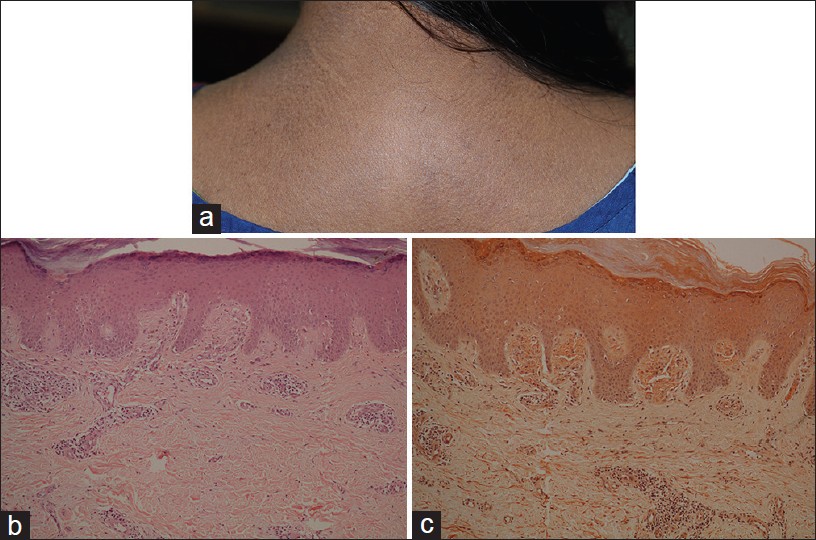 |
| Figure 11: Macular amyloidosis (a) Reticulate hyperpigmentation, upper back, (b) Distended dermal papillae filled with eosinophilic material (H and E, ×100), (c) Orange-pink amyloid (Congo red stain, ×100) |
Histology shows small, sometimes subtle, globular deposits of eosinophilic amyloid and melanophages, confined within a few distended dermal papillae. Additional features include sparse vacuolar damage and apoptotic keratinocytes. [34] Amyloid is highlighted by Congo red and crystal violet stains [Figure - 11]b and c. Amyloid deposits are focal and may be small enough to escape detection in the biopsy.
Fixed Drug Eruption
Fixed drug eruption (FDE) is clinically distinctive. It presents as recurrent plaque(s) at fixed locations, commonly lips, genitalia, and acral areas subsequent to each exposure to the offending drug. Fixed drug eruption (FDE) plaques are erythema multiforme (EM)-like, even targetoid, usually non-itchy and resolve spontaneously with postinflammatory hyperpigmentation (PIH). Less commonly, FDE may present as pigmented macules. Many drugs are implicated in FDE, some notable being barbiturates, ibuprofen, and sulfonamides.
Histologically, vacuolar type of interface pathology observed is associated with necrotic keratinocytes and lymphocytic infiltrate that obscures the dermo epidermal junction (DEJ). Pigment incontinence is a prominent feature. Subepidermal vesiculation may be seen. Upper dermal inflammation consists of lymphohistiocytes, neutrophils, and eosinophils. Dermal melanophages may be the only tell-tale sign of a resolved lesion of FDE. [35]
Confluent Reticulate Papillomatosis of Gougerot-Carteaud
Confluent reticulate papillomatosis (CRP) is an uncommon pigmentary disorder characterized by asymptomatic, persistent, coalescing brown papules, and small plaques forming a characteristic reticulate pattern. It has predilection for upper chest, upper back, shoulders, and neck, and is bilaterally symmetrical [36] [Figure - 12]a.
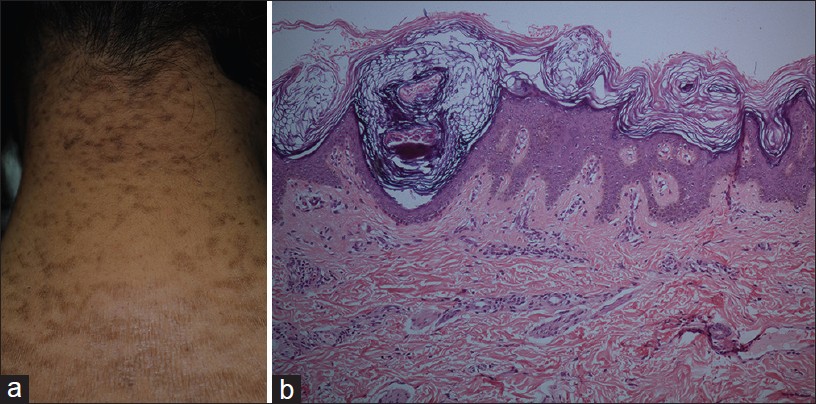 |
| Figure 12: Confluent reticulate papillomatosis (a) Coalescing brown papules and small plaques forming reticulate pattern, nape of neck. (b) Papillomatosis, acanthosis at the base of valleys, covered by basket weave keratin (H and E, ×100) |
Histopathology shows a low papillomatous lesion covered by basket weave keratin. Epidermis is acanthotic at the base of the valleys between the papillomatous areas. Mild basal hyperpigmentation may be apparent in some cases [Figure - 12]b. The pathology of CRP overlaps with mild forms of AN.
Becker′s Nevus
Becker′s nevus (melanosis) is an acquired melanosis that presents in late childhood or early adolescence. A hyperpigmented patch gradually evolves into a plaque and usually displays variable degree of hypertrichosis. It typically arises in the vicinity of the large joints especially shoulders, elbows, and hips. Rarely, multiple sites may be affected. [37] Once fully evolved, Becker′s nevus persists unchanged through life.
Histopathologically, Becker′s nevus shows both epidermal and dermal abnormalities. The epidermal changes include acanthosis, mild papillomatosis, and variable basal hyperpigmentation. The rate ridges in Becker′s nevus are flat in contradistinction to epidermal nevus. Dermal changes include increased number of hair follicles and sebaceous glands. Hypertrophy of arrector pilorum muscle along with bundles of smooth muscle fibers independent of adnexa are the distinctive pathological features. Dermal melanophages are also noted. [37]
Ephelides (Freckles)
Ephelides are small pigmented macules that occur on the face and less commonly on other sun-exposed areas of light complexioned individuals. They are increasingly being observed in Indians with skin type IV and V. Ephelides are irregular, discrete, small macules, about 1-2 mm in size, typically distributed over the nose and malar areas. The pigmentation varies corresponding to seasons and level of sun exposure. [38]
Histological features show hypermelanization of basal epidermis with otherwise normal epidermal architecture. There is no increase in the number of melanocytes. Electron microscopy studies have shown increased melanin production by the affected melanocytes in contrast to normal skin. [39]
Psoralens and ultraviolet-A light (PUVA) therapy [40] is known to stimulate melanogenesis and causes ephelide-like pigmentation in some individuals which histologically is indistinguishable from classic ephelides. Cafι-au-lait spots also share the same pathological features.
Conclusion
Disorders of hyperpigmentation discussed above focus on the acquired diseases and cover the most common diagnoses and demonstrate the utility of histopathology as the clinical presentations overlap significantly. Facial melanoses are group of heterogenous disorders, mistaken clinically as melasma The benefit from histopathological evaluation, is not only to confirm but exclude related disorders because management of these differs significantly. Discoid lupus erythematosus requires systemic therapy, as does LPP and AN. Some conditions are extremely resistant to treatment such as exogenous ochronosis and argyria, but cessation of the causative factors can halt progression of the disease. Targeted diagnosis and therapy can prevent progression of disease and years of failed treatments in patients. More importantly, it can reveal potentially disfiguring skin diseases such as DLE or highlights metabolic disease such as in AN.
This review, of most common acquired disorders, highlights the value of histopathology in evaluating hyperpigmentary disorders that afflicts so many of Indians seeking medical assistance. The key histopathological features are highlighted with emphasis on differential diagnosis that come into consideration. In conclusion, a specific diagnosis of hyperpigmentary disorder is possible based on histopathological findings and its interpretation in the context of clinical presentation.
| 1. |
Khanna N, Rasool S. Facial melanoses: Indian perspective. Indian J Dermatol Venereol Leprol 2011;77:552-63.
[Google Scholar]
|
| 2. |
Sarkar R, Puri P, Jain RK, Singh A, Desai A. Melasma in men: A clinical, aetiological and histological study. J Eur Acad Dermatol Venereol 2010;24:768-72.
[Google Scholar]
|
| 3. |
Sanchez NP, Pathak MA, Sato S, Fitzpatrick TB, Sanchez JL, Mihm MC Jr. Melasma: A clinical, light microscopic, ultrastructural, and immunofluorescence study. J Am Acad Dermatol 1981;4:698-710.
[Google Scholar]
|
| 4. |
Grimes PE, Yamada N, Bhawan J. Light microscopic, immunohistochemical, and ultrastructural alterations in patients with melasma. Am J Dermatopathol 2005;27:96-101.
[Google Scholar]
|
| 5. |
Kang WH, Yoon KH, Lee ES, Kim J, Lee KB, Yim H, et al. Melasma: Histopathological characteristics in 56 Korean patients. Br J Dermatol 2002;146:228-37.
[Google Scholar]
|
| 6. |
Bhutani LK, Bedi TR, Pandhi RK, Nayak NC. Lichen planus pigmentosus. Dermatologica 1974;149:43-50.
[Google Scholar]
|
| 7. |
Kanwar AJ, Dogra S, Handa S, Parsad D, Radotra BD. A study of 124 Indian patients with lichen planus pigmentosus. Clin Exp Dermatol 2003;28:481-5.
[Google Scholar]
|
| 8. |
Ramirez CO. Dermatosis cenicienta: Estudio epidemiologico de 139 casos. Dermatol Rev Mex 1966;10:133-42.
[Google Scholar]
|
| 9. |
Pock L, Jelínková L, Drlík L, Abrhámová S, Vojtechovská S, Sezemská D, et al. Lichen planus pigmentosus-inversus. J Eur Acad Dermatol Venereol 2001;15:452-4.
[Google Scholar]
|
| 10. |
George R, Mathai R, Kurian S. Cutaneous lupus erythematosus in India: Immunofluorescence profile. Int J Dermatol 1992;31:265-9.
[Google Scholar]
|
| 11. |
Aberer E. Lupus erythematosus. Wide range of symptoms through clinical variation, associated diseases and imitators. Hautarzt 2010;61:676-82.
[Google Scholar]
|
| 12. |
Job CK, Yoder L, Jacobson RR, Hastings RC. Skin pigmentation from clofazimine therapy in leprosy patients: A reappraisal. J Am Acad Dermatol 1990;23:236-41.
[Google Scholar]
|
| 13. |
Kossard S, Doherty E, McColl I, Ryman W. Auto fluorescence of clofazimine in discoid lupus erythematosus. J Am Acad Dermatol 1987;17:867-71.
[Google Scholar]
|
| 14. |
Lansdown AB. A pharmacological and toxicological profile of silver as an antimicrobial agent in medical devices. Adv Pharmacol Sci 2010;2010:910686.
[Google Scholar]
|
| 15. |
Brandt D, Park B, Hoang M, Jacobe HT. Argyria secondary to ingestion of homemade silver solution. J Am Acad Dermatol 2005;53:S105-7.
[Google Scholar]
|
| 16. |
White JM, Powell AM, Brady K, Russell-Jones R. Severe generalized argyria secondary to ingestion of colloidal silver protein. Clin Exp Dermatol 2003;28:254-6.
[Google Scholar]
|
| 17. |
Utikal J, Thoelke A, Becker JC, Figl R, Goerdt S, Schadendorf D, et al. Local cutaneous argyria mimicking melanoma metastases in a patient with disseminated melanoma. J Am Acad Dermatol 2006;55:S92-4.
[Google Scholar]
|
| 18. |
Rackoff EM, Benbenisty KM, Maize JC, Maize JC Jr. Localized cutaneous argyria from an acupuncture needle clinically concerning for metastatic melanoma. Cutis 2007;80:423-6.
[Google Scholar]
|
| 19. |
Pezzarossa E, Alinovi A, Ferrari C. Generalized argyria. J Cutan Pathol 1983;10:361-3.
[Google Scholar]
|
| 20. |
Bleehen SS, Gould DJ, Harrington CI, Durrant TE, Slater DN, Underwood JC. Occupational argyria; light and electron microscopic studies and X-ray microanalysis. Br J Dermatol 1981;104:19-26.
[Google Scholar]
|
| 21. |
Phillips JI, Isaacson C, Carman H. Ochronosis in black South Africans who used skin lighteners. Am J Dermatopathol 1986;8:14-21.
[Google Scholar]
|
| 22. |
Albers SE, Brozena SJ, Glass LF, Fenske NA. Alkaptonuria and ochronosis: Case report and review. J Am Acad Dermatol 1992;27:609-14.
[Google Scholar]
|
| 23. |
Puri N. A study of pathogenesis of acanthosis nigricans and its clinical implications. Indian J Dermatol 2011;56:678-83.
[Google Scholar]
|
| 24. |
Hall JM, Moreland A, Cox GJ, Wade TR. Oral acanthosisnigricans: Report of a case and comparison of oral and cutaneous pathology. Am J Dermatopathol 1988;10:68-73.
[Google Scholar]
|
| 25. |
Katoulis AC, Stavrianeas NG, Georgala S, Bozi E, Kalogeromitros D, Koumantaki E, et al. Poikiloderma of Civatte: A clinical and epidemiological study. J Eur Acad Dermatol Venereol 2005;19:444-8.
[Google Scholar]
|
| 26. |
Katoulis AC, Stavrianeas NG, Katsarou A, Antoniou C, Georgala S, Rigopoulos D, et al. Evaluation of the role of contact sensitization and photosensitivity in the pathogenesis of poikiloderma of Civatte. Br J Dermatol 2002;147:493-7.
[Google Scholar]
|
| 27. |
Nakayama H, Harada R, Toda M. Pigmented cosmetic dermatitis. Int J Dermatol 1976;15:673-5.
[Google Scholar]
|
| 28. |
Nath AK, Thappa DM. Kumkum-induced dermatitis: An analysis of 46 cases. Clin Exp Dermatol 2007;32:385-7.
[Google Scholar]
|
| 29. |
Shenoi SD, Rao R. Pigmented contact dermatitis. Indian J Dermatol Venereol Leprol 2007;73:285-7.
[Google Scholar]
|
| 30. |
Serrano G, Pujol C, Cuadra J, Gallo S, Aliaga A. Riehl's melanosis: Pigmented contact dermatitis caused by fragrances. J Am Acad Dermatol 1989;21:1057-60.
[Google Scholar]
|
| 31. |
Hori Y, Kawashima M, Oohara K, Kukita A. Acquired, bilateral nevus of Ota-like macules. J Am Acad Dermatol 1984;10:961-4.
[Google Scholar]
|
| 32. |
Lee JY, Kim EH, Kim KH, Kang HY, Lee ES, Kim YC. Acquired bilateral naevus of Ota-like macules: An immunohistological analysis of dermal melanogenic paracrine cytokine networks. Br J Dermatol 2011;164:580-5.
[Google Scholar]
|
| 33. |
Park JM, Tsao H, Tsao S. Acquired bilateral nevus of Ota-like macules (Hori nevus): Etiologic and therapeutic considerations. J Am Acad Dermatol 2009;61:88-93.
[Google Scholar]
|
| 34. |
Kibbi AG, Rubeiz NG, Zaynoun ST, Kurban AK. Primary localized cutaneous amyloidosis. Int J Dermatol 1992;31:95-8.
[Google Scholar]
|
| 35. |
Sehgal VN, Gangwani OP. Fixed drug eruption. Current concepts. Int J Dermatol 1987;26:67-74.
[Google Scholar]
|
| 36. |
Davis MD, Weenig RH, Camilleri MJ. Confluent and reticulate papillomatosis (Gougerot-Carteaud syndrome): A minocycline-responsive dermatosis without evidence for yeast in pathogenesis. A study of 39 patients and a proposal of diagnostic criteria. Br J Dermatol 2006;154:287-93.
[Google Scholar]
|
| 37. |
Khaitan BK, Manchanda Y, Mittal R, Singh MK. Multiple Becker's naevi: A rare presentation. Acta Derm Venereol 2001;81:374-5.
[Google Scholar]
|
| 38. |
Ber Rahman S, Bhawan J. Lentigo. Int J Dermatol 1996;35:229-39.
[Google Scholar]
|
| 39. |
Breathnach AS, Wyllie LM. Electron microscopy of melanocytes and melanosomes in freckled human epidermis. J Invest Dermatol 1964;42:389-94.
[Google Scholar]
|
| 40. |
Maize JC, Ackerman AB. Freckle. In: Maize JC, Ackerman AB, editors. Pigmented lesions of the skin. Philadelphia: Lea and Febiger; 1987. p. 28-30.
[Google Scholar]
|
Fulltext Views
13,120
PDF downloads
4,241





