Translate this page into:
Clinicopathological features and prognosis of pyoderma gangrenosum in Korea: A single centre, retrospective, observational study over 20 years
Corresponding author: Woo Jin Lee, Department of Dermatology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, South Korea. uucm79@hanmail.net
-
Received: ,
Accepted: ,
How to cite this article: Kim YJ, Lee KH, Won CH, Chang SE, Lee MW, Choi JH, et al. Clinicopathological features and prognosis of pyoderma gangrenosum in Korea: A single centre, retrospective, observational study over 20 years. Indian J Dermatol Venereol Leprol 2023;89:25-34.
Abstract
Background:
Pyoderma gangrenosum is a rare autoinflammatory neutrophilic dermatosis that rapidly evolves. However, little is known about the clinicopathological features and prognosis of pyoderma gangrenosum.
Aims:
We aimed to document clinicopathologic and prognostic data of the patients with pyoderma gangrenosum.
Methods:
In this retrospective observational study, we reviewed case records of patients diagnosed with pyoderma gangrenosum between 1999–2019.
Results:
Fifty-three patients were identified by reviewing medical records for skin biopsy; of these, 37 were men and 16 were women. Mean age at onset was 43.3 ± 18.5 years. The most frequently affected area was the lower extremities (60.4%), followed by the head and neck (17.0%). The most common subtype was ulcerative (47.2%), followed by bullous (22.6%). 30 cases had underlying diseases and the most common were malignancy (24.5%), followed by inflammatory bowel diseases (18.9%). The proportion of cases with history of trauma were significantly higher in post-operative type (100%) as compared to the bullous type (8.3%). Histologic features of granulation tissue were frequently found in post-operative type (66.7%) and bullous type (58.3%). Granulomas were predominantly found in bullous type (58.3%). Age <60 years appeared to be significantly associated with multiple lesions. Partial-to-complete remission was observed in 40 cases (75.5%). Nine (17.0%) cases experienced recurrence with a median progression-free period of six months (interquartile range of 3.0–9.0 months). Cases with underlying hematologic disorders and the bullous subtype were significantly associated with early recurrence.
Limitations:
This study was a single-centre study with a retrospective design.
Conclusion:
Pyoderma gangrenosum appears to have ethnic differences. Underlying haematologic disorders and bullous subtype have a worse prognosis. However, the type of histopathology did not correlate with the clinical outcome of pyoderma gangrenosum.
Keywords
Pyoderma gangrenosum
inflammation
skin
Asia
Plain Language Summary
Pyoderma gangrenosum is an inflammatory skin disease, resulting from a process by which the body’s immune system malfunctions. It may progress aggressively unless early treatment is administered. However, early diagnosis is difficult because other diseases need to be ruled out. The incidence of the disease is in 3–10 patients per million population per year in Western countries. However, there have been lack of reports on Asian cases of pyoderma gangrenosum and limited data is available on treatment outcomes. In this observational study, the authors aimed to document clinical and microscopic features of all patients diagnosed with pyoderma gangrenosum between the years of 1999 to 2019. By reviewing medical records, 53 cases of this disease were detected, of which 37 were men and 16 were women. Their mean age was 43.3±18.5 years. The most frequently affected area was the lower legs (60.4%). Thirty patients had other diseases and the most common were cancers (24.5%). Nine (17.0%) patients experienced new occurrence of pyoderma gangrenosum after a mean period of 6.87±18.5 months. The type of microscopic features did not correlate with the clinical outcome. Patients with underlying haematologic disease (which primarily affect the blood and blood-forming organs) and pyoderma gangrenosum presenting as fluid-filled lesions showed earlier new occurrence of disease than others.
Introduction
Pyoderma gangrenosum is a rare, non-infectious inflammatory neutrophilic dermatosis and often presents as painful ulcers.1 Ever since Brunsting et al. renamed this entity as “pyoderma gangrenosum,”2 many cases have been reported, warning about its rapid and aggressive course. However, even at present, new information on the disease is helping to update knowledge of this entity. The estimated incidence of pyoderma gangrenosum in western countries is 3–10 patients per million population per year.3 There are various clinical manifestations of pyoderma gangrenosum, with five types of pyoderma gangrenosum having been identified: classic (ulcerative), bullous, pustular, vegetative and post-operative.4 Nonetheless, few diagnostic pathologic features have been described in this entity. Due to its clinical variance and lack of reports, clinicians often face diagnostic difficulties with pyoderma gangrenosum because its diagnosis is based on the exclusion of other diseases.
Early immunosuppressant treatment can halt aggressive progression and thus, early diagnosis by awareness of pyoderma gangrenosum is essential. However, limited data is available on treatment outcomes and prognosis of pyoderma gangrenosum. There has been a paucity of reports on Asian cases of pyoderma gangrenosum as well, despite its possible ethnic differences.
In this study, we evaluated the clinical and pathologic characteristics of 53 patients with pyoderma gangrenosum. Treatment responses to various therapeutic agents were comprehensively reviewed. Clinical outcomes were also analysed to investigate the prognostic factors of pyoderma gangrenosum.
Methods
Patients were selected after retrospectively reviewing patient charts at Asan Medical Center (Seoul, South Korea) between March 1999 and December 2019. The institutional review board of the Asan Medical Center approved this study. Written informed consent was obtained from all patients. We aimed to investigate clinicopathologic and prognostic data, along with therapeutic response in patients with pyoderma gangrenosum. We included patients with a definitive diagnosis confirmed through clinicohistopathologic examination. Two different dermatopathologists (YJK and KHL) confirmed the exclusion of other histopathological diagnoses using uniform criteria. Since there are no specific diagnostic tests for pyoderma gangrenosum, proposed diagnostic criteria were used [Box 1]. Discrepancies were resolved by joint evaluation. For cases where information was not well described, three dermatologists (YJK, KHL and WJL) who participated in the study reviewed clinical data together and decided whether to include them, according to the majority opinion. From the medical records, we collected data on age, sex, onset, size and location of lesions, trauma history, symptoms and signs, clinical subtypes, underlying diseases, treatment and outcome. We defined delayed cases as all cases in which pyoderma gangrenosum was not the first top three diagnostic impressions in the clinicians’ initial examination. Multiple lesions were defined as those with two or more lesions rather than a single one. When accompanied by clear serous material, it was documented as oozing. Active bleeding or malodorous discharge was not considered oozing. With regard to histologic analysis, we defined a granuloma as the proliferation and focal aggregation of histiocytic cells. Neutrophilic infiltration was classified into absent, mild, moderate and severe.5
Response to treatment was evaluated one month after initial treatment, as either a complete remission with extinction of lesions, partial remission with improvement, stable disease without improvement or aggravation and progression of disease state with aggravation and/or change to second line treatment because of insufficient response to first line treatment. Progression-free period was measured from the date of diagnosis until the date of local or regional aggravation or recurrence on last follow-up. Recurrence was defined as occurrence of lesions two weeks or more after being confirmed as complete remission. The Kaplan–Meier method was used to plot survival curves and a Cox proportional hazard model was used to identify the factors associated with progression-free period. Pearson’s Chi-square test and Fisher’s exact test was used to compare the categorical variables. One-way analysis of variance and Kruskal-Wallis test with post hoc was performed to compare the continuous variables among different subtypes. All data were statistically analysed using SPSS version 18.0 (SPSS Inc., Chicago, IL); P < 0.05 was considered to be statistically significant.
Results
Clinicopathological characteristics of pyoderma gangrenosum
A total of 76,080 skin biopsy reports were reviewed and 53 (0.07%) cases of pyoderma gangrenosum were found between March 1999 and December 2019, of which 37 were men and 16 were women [Table 1]. Patient ages at initial diagnosis ranged from 12 to 81 years (mean ± standard deviation: 43.3 ± 18.5 years). The period of onset to diagnosis ranged from three days to 60 months (mean ± SD 6.91 ± 14.5 months). The mean size of skin lesion was 5.09 ± 4.13 cm. There were 25 cases (47.2%) of the ulcerative type, 12 cases (22.6%) of the bullous type, 11 cases (20.7%) of the vegetative type and two cases (3.8%) of the pustular type [Figure 1]. Three cases (5.7%) were of the post-operative type, all occurring after total colectomy with ileostomy, resection of small intestine and right lower lobe wedge resection.
| Characteristics | Pyoderma gangrenosum |
|---|---|
| Total patients, n | 53 |
| Age, year (mean±standard | 43.3±18.5 |
| deviation) | |
| Sex ratio (male: female) | 37 (69.8%):16 (30.2%) |
| Onset to diagnosis (month) | 6.91±14.5 |
| Delayed diagnosis | 14 (26.4%) |
| Mean size (cm) | 5.09 |
| Location | Lower extremities 32 (60.4%) Head and neck 9 (17.0%) Trunk 8 (15.1%) Upper extremities 4 (7.5%) |
| Multiple lesions | 27 (50.9%) |
| Subtype | Ulcerative 25 (47.2%) Bullous 12 (22.6%) Vegetative 11 (20.7%) Pustular 2 (3.8%) Post-operative 3 (5.7%) |
| Signs | Ulcer 47 (88.7%) Bullae 16 (30.2%) Pustule 7 (13.2%) Plaque and papule 7 (13.2%) Patch 5 (9.4%) Crust 5 (9.4%) |
| Symptoms | Pain and tenderness 42 (79.2%) Oozing 19 (35.8%) Itching sensation 7 (13.2%) Burning sensation 2 (3.8%) Oedema 1 (1.9%) |
| Medical history | Haematologic malignancy 8 (15.1%) Myelodysplastic syndrome 5 (9.4%) Chronic myelomonocytic leukaemia 1 (1.9%) Acute myeloid leukaemia 1 (1.9%) Aplastic anaemia 1 (1.9%) Other solid organ cancers 5 (9.4%) Early gastric cancer 4 (7.5%) Ovarian cancer 1 (1.9%) Inflammatory bowel disease 10 (18.9%) Crohn’s disease 6 (11.3%) Ulcerative colitis 4 (7.5%) Aortic aneurysm or dissection 3 (5.7%) Behcet’s diseases 2 (3.8%) Rheumatoid arthritis 2 (3.8%) Autoimmune pancreatitis 1 (1.9%) Primary sclerosing cholangitis 1 (1.9%) Spondyloarthropathy 1 (1.9%) |
| Trauma history | 20 (37.7%) |
| Initial treatment | Systemic steroid 22 (41.5%) Complete remission 1 (4.5%) Partial remission 14 (63.6%) Treatment related side effects |
| Leukocytosis 2 (9.1%) Dapsone 5 (9.4%) Complete remission 0 Partial remission 1 (20.0%) |
|
| Systemic antibiotics 12 (22.6%) Complete remission 1 (8.3%) Partial remission 1 (8.3%) Topical antibiotics 9 (17.0%) Complete remission 0 Partial remission 0 Topical tacrolimus 3 (5.7%) Complete remission 1 (33.3%) Partial remission 0 Treatment related side effects Local pain 1 (33.3%) Antihistamine 2 (3.7%) Complete remission 0 Partial remission 1 (50.0%) |
|
| Additional treatment | Systemic steroid 28 (52.8%) Complete remission 2 (7.1%) Partial remission 17 (60.7%) Treatment related side effects Leukocytosis 2 (7.1%) Topical steroid 4 (7.5%) Complete remission 1 (25.0%) Partial remission 2 (50.0%) Systemic antibiotics 15 (28.3%) Complete remission 1 (6.7%) Partial remission 2 (13.3%) Topical antibiotics 33 (62.3%) Complete remission 0 Partial remission 11 (33.3%) Dapsone 8 (15.1%) Complete remission 0 Partial remission 3 (37.5%) Topical tacrolimus 4 (7.5%) Complete remission 1 (25.0%) Partial remission 0 Treatment related side effects Local pain 1 (25.0%) Colchicine 3 (5.7%) Complete remission 1 (33.3%) Partial remission 0 Treatment related side effects Diarrhea 1 (33.3%) Methotrexate 2 (3.8%) Complete remission 1 (50.0%) Partial remission 0 Cyclosporine 2 (3.8%) Complete remission 1 (50.0%) Partial remission 0 Steroid intra-lesional injection 1 (1.9%) Complete remission 0 Partial remission 0 Treatment related side effects Local skin atrophy 1 (100%) |
| Debridement | 10 (18.9%) |
| Recurrence 3 (30.0%) | |
| Follow-up duration (month) | 7.72±18.5 |
| Follow-up loss: 17 (32.1%) | |
| Progression-free survival (month) | Mean±standard deviation: 6.69±17.6 Median, interquartile range: 1.0, 1.0–5.0 |
| Recurrence | 9 (17.0%) |
| Death | 8 (15.1%) |

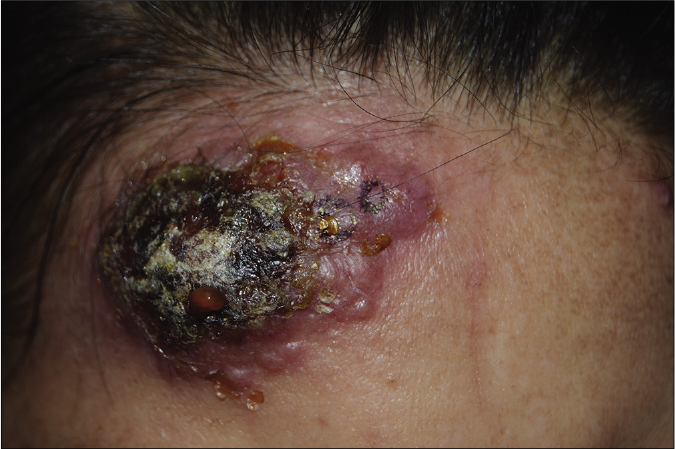
- Ulcerative type, which is the most common form of pyoderma gangrenosum, showing a rapidly enlarged deep ulceration with erythematous borders
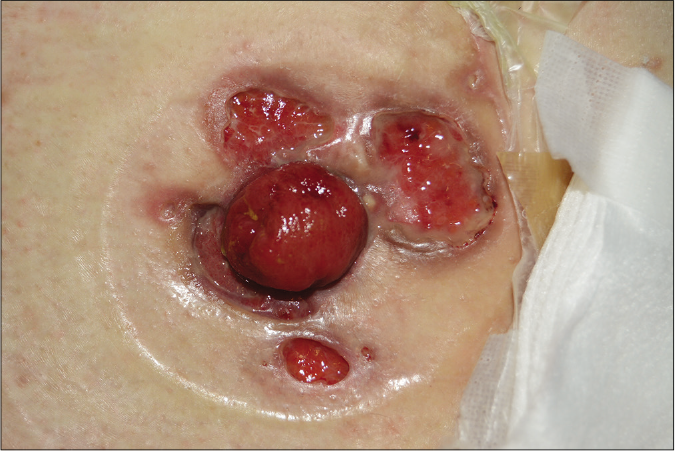
- Bullous pyoderma gangrenosum, a rapidly developed bulla with crusts and central necrosis
- Pustular pyoderma gangrenosum, a rare type of pyoderma gangrenosum presenting with grouped pustular eruption
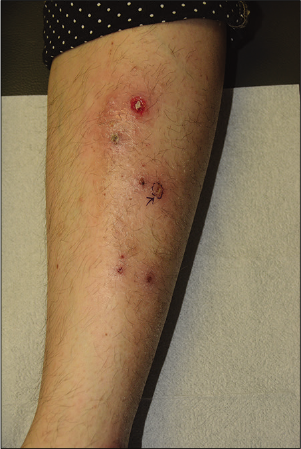
- Vegetative pyoderma gangrenosum, a superficial and non-aggressive type of pyoderma gangrenosum, showing shallow erosion
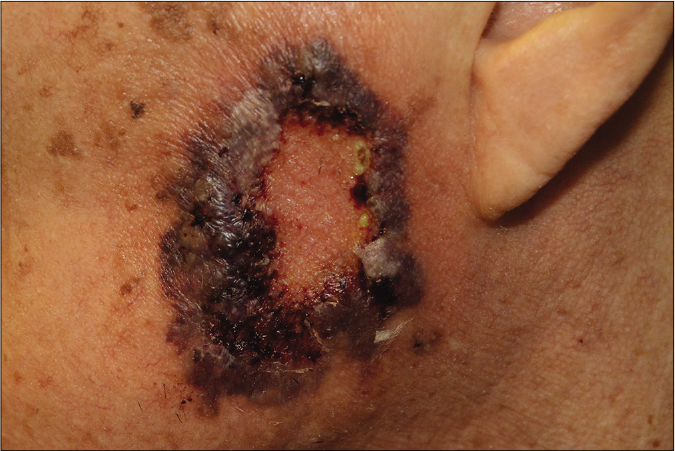
- Post-operative pyoderma gangrenosum, an atypical type of pyoderma gangrenosum located on the fistulous tracts and the surrounding areas
Lower extremities were the most affected site (n = 32, 60.4%). Pain/tenderness (n = 42, 79.2%) was the most common initial symptom/sign of skin lesion. Twenty cases (37.7%) had an antecedent history of trauma at the site of the skin lesion. Eight cases (15.1%) had underlying haematologic disorders. Five cases (9.4%) had fever at the time of initial diagnosis. Ulcerative colitis was observed in four cases (7.5%) and Crohn’s disease in six cases (11.3%). Associated malignancies were found in five cases (9.4%), most commonly in early gastric cancer (n = 4, 7.5%) followed by ovarian cancer (n = 1, 1.9%).
When we analysed histopathologic characteristics, all cases revealed neutrophil infiltrations at various levels [Figure 2]. However, the degree of neutrophilic infiltration was not significantly different in the subtypes of pyoderma gangrenosum (P = 0.277). Epidermal ulceration (n = 33, 62.3%) was the second most common feature of pyoderma gangrenosum. However, these features were not significantly correlating with clinical outcomes.
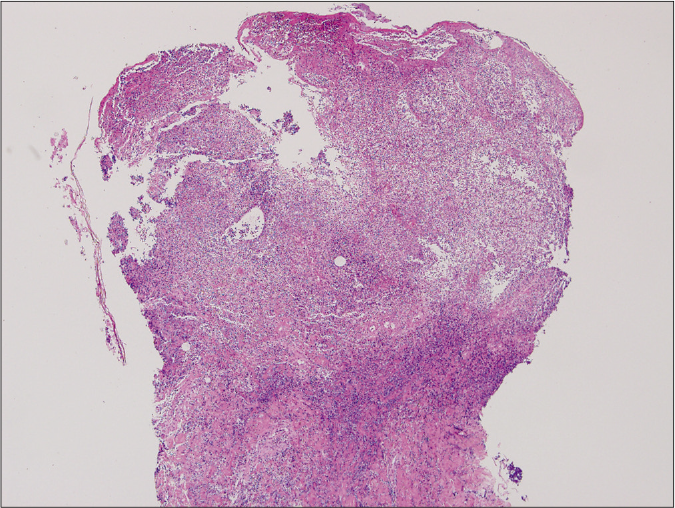
- Ulcerative type revealed diffuse epidermal necrotic ulcer with neutrophilic infiltrations and dermal granulation tissue (haematoxylin and eosin stain, ×125)
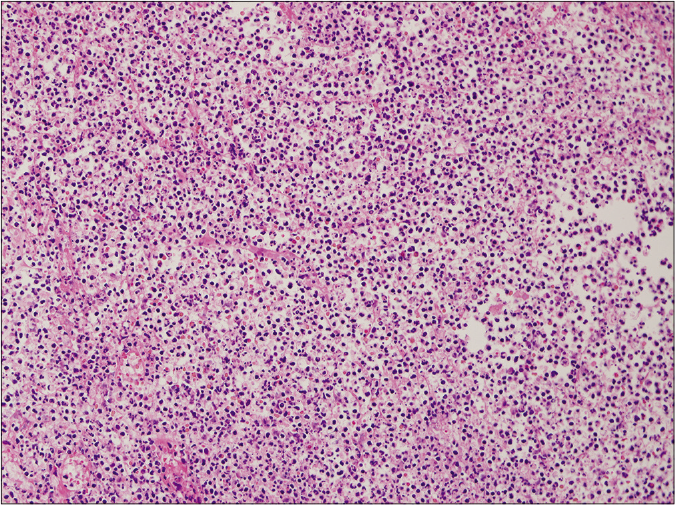
- Ulcerative type, on high power (haematoxylin and eosin stain, ×2000)
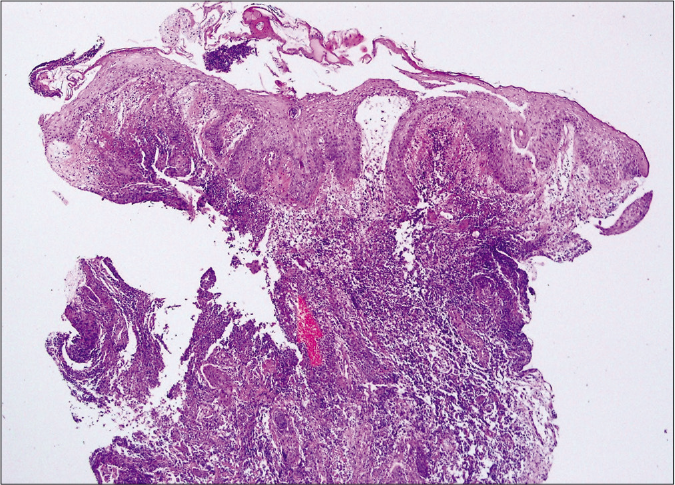
- Bullous type showed epidermal ulcers with subepidermal bulla and upper dermal oedema. Diffuse dermal massive neutrophilic and eosinophilic infiltrations were also noted. (haematoxylin and eosin stain, ×400)
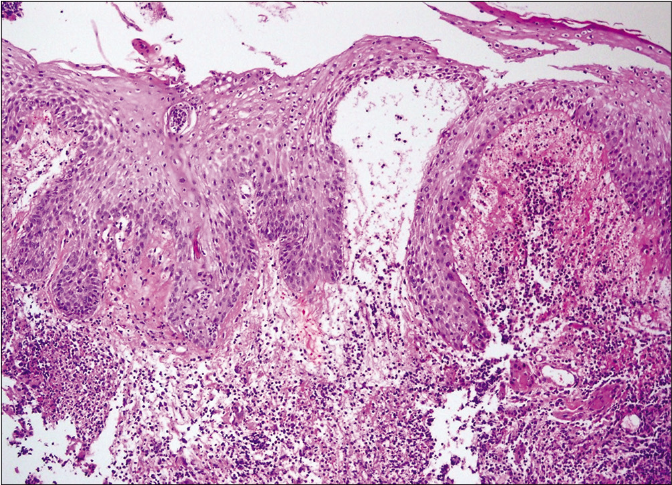
- Bullous type on higher magnification (haematoxylin and eosin stain, ×1000)
Comparative clinicopathologic subgroup analysis
Of the 53 patients diagnosed with pyoderma gangrenosum, comparative subgroup analysis was performed. The presence of trauma history was significantly different among subtypes (P = 0.023), being highest in the ulcerative variety, whereas other clinical variables were not different among subtypes [Table 2]. Histopathologic analysis showed that the presence of granulation tissue and granuloma was significantly different among subtypes (P = 0.012) with 7 of 12 patients (58.3%) with bullous type exhibiting these [Table 2]. There was no statistical difference among subgroups regarding other histopathologic features.
| Clinical features | Ulcerative (n=25) | Bullous (n=12) | Vegetative (n=11) | Pustular (n=2) | Post-operative (n=3) | P-value | |
|---|---|---|---|---|---|---|---|
| Age, year (mean±SD) | 44.2±17.7 | 50.8±16.5 | 34.5±19.2 | 54.5±34.6 | 30.0±8.67 | 0.145 | |
| (Age≥60: Age<60) | (6:19) | (4:8) | (2:9) | (1:1) | (0:3) | 0.659 | |
| Sex ratio (male: female) | 16:9 (64%:36%) | 9:3 (75%:25%) | 8:3 (73%:27%) | 2:0 (100%:0%) | 2:1 (67%:33%) | 0.911 | |
| Mean size of initial lesion (cm) | 5.32±4.36 | 5.89±5.24 | 3.91±2.42 | 4.50±0.70 | 4.67±4.61 | 0.829 | |
| Duration of skin lesion (months) | 10.70±20.93 | 3.25±5.66 | 9.54±21.03 | 1.62±1.94 | 1.16±0.28 | 0.701 | |
| Time to diagnosis (months) | 9.74±18.40 | 2.92±4.76 | 7.36±13.95 | 1.62±1.94 | 1.16±0.28 | 0.639 | |
| Delayed diagnosis (%) | 9 (36.0) | 2 (16.7) | 3 (27.3) | 0 | 0 | 0.588 | |
| Location (%) | |||||||
| Lower extremities | 19 (76.0) | 8 (66.7) | 4 (36.4) | 1 (50.0) | 0 | 0.120 | |
| Head and neck | 3 (12.0) | 1 (8.3) | 4 (36.4) | 1 (50.0) | 0 | 0.127 | |
| Trunk | 0 | 2 (16.7) | 3 (27.3) | 0 | 3 (100.0) | 0.063 | |
| Upper extremities | 3 (12.0) | 1 (8.3) | 0 | 0 | 0 | 0.817 | |
| Multiple lesions (%) | 13 (52.0) | 8 (66.7) | 4 (36.4) | 0 | 2 (66.7) | 0.393 | |
| Trauma history (%) | 10 (40.0) | 1 (8.3) | 5 (45.5) | 1 (50.0) | 3 (100.0) | 0.023* | |
| Past medical history (%) | |||||||
| Haematologic malignancy | 2 (8.0) | 4 (33.3) | 1 (9.1) | 0 | 1 (33.3) | 0.181 | |
| Other solid organ cancers | 2 (8.0) | 2 (16.7) | 0 | 1 (50.0) | 0 | 0.329 | |
| Inflammatory bowel disease | 6 (24.0) | 2 (16.7) | 1 (9.1) | 0 | 1 (33.3) | 0.754 | |
| Aortic aneurysm or dissection | 2 (8.0) | 1 (8.3) | 0 | 0 | 0 | 0.923 | |
| Behcet’s disease | 2 (8.0) | 0 | 0 | 0 | 0 | 0.782 | |
| Rheumatoid arthritis | 0 | 1 (8.3) | 1 (9.1) | 0 | 0 | 0.317 | |
| Autoimmune pancreatitis | 1 (4.0) | 0 | 0 | 0 | 0 | 0.764 | |
| Primary sclerosing cholangitis | 0 | 1 (8.3) | 0 | 0 | 0 | 0.415 | |
| Spondyloarthropathy | 1 (4.0) | 0 | 0 | 0 | 0 | 0.764 | |
| Histopathologic features (%) | |||||||
| Epidermal ulceration | 14 (56.0) | 9 (75.0) | 6 (54.5) | 1 (50.0) | 3 (100) | 0.514 | |
| Perivascular infiltration | 8 (32.0) | 7 (58.3) | 2 (18.2) | 0 | 0 | 0.177 | |
| Vasculitis | 4 (16.0) | 1 (8.3) | 3 (27.2) | 0 | 0 | 0.768 | |
| Granulation tissue | 4 (16.0) | 7 (58.3) | 1 (9.1) | 0 | 2 (66.7) | 0.012* | |
| Granuloma | 2 (8.0) | 7 (58.3) | 2 (18.2) | 0 | 0 | 0.012* | |
| Haemorrhage | 4 (16.0) | 3 (25.0) | 1 (9.1) | 0 | 0 | 0.865 | |
| Pseudoepitheliomatous hyperplasia | 4 (16.0) | 4 (33.3) | 1 (9.1) | 0 | 0 | 0.539 | |
| Neutrophil infiltration | 0.277 | ||||||
| Minimal | 5 (20.0) | 1 (8.3) | 4 (36.4 | 1 (50.0) | 1 (33.3) | ||
| Mild | 10 (40.0) | 9 (75.0) | 2 (18.2) | 1 (50.0) | 1 (33.3) | ||
| Moderate | 6 (24.0) | 0 | 3 (27.2) | 0 | 0 | ||
| Severe | 4 (16.0) | 2 (16.7) | 2 (18.2) | 0 | 1 (33.3) | ||
| Lymphocyte infiltration | 4 (16.0) | 1 (8.3) | 3 (27.2) | 0 | 0 | 0.686 | |
| Plasma cell infiltration | 1 (4.0) | 1 (8.3) | 2 (18.2) | 0 | 0 | 0.498 | |
| Eosinophil infiltration | 1 (4.0) | 4 (33.3) | 0 | 0 | 0 | 0.079 | |
| Mixed cell inflammation | 7 (28.0) | 5 (41.6) | 4 (36.4) | 1 (50.0) | 0 | 0.678 | |
*P<0.05 were considered to be statistically significant. SD: Standard deviation
The subgroup aged <60 years presented with multiple lesions more commonly as compared to others [P = 0.004, Table 2]. Underlying malignancy was also more frequent in this subgroup (P = 0.005). Other clinical variables including progression-free period were not significantly different. When we further subdivided the patients into smaller age groups of 20-year units for further analysis, involvement of the upper extremities (P = 0.007), multiple skin lesions (P = 0.002) and underlying malignancy (P = 0.008) was significantly different. Since 37 out of 53 patients (69.8%) were male, a subgroup analysis was also performed between men and women; however, no statistically significant difference in clinical features was observed.
Treatment outcome to variable therapeutic agents
Of the 53 patients diagnosed with pyoderma gangrenosum, 22 cases (41.5%) received steroids as initial treatment and 15 of those cases (68.2%) showed at least a partial response. Treatment response and related side effects to each of the main treatment modalities are summarised in Table 1.
Clinical course of pyoderma gangrenosum according to their clinicopathologic characteristics
A total of 53 patients were included, while 17 patients (32.1%) were lost to follow-up. Forty-five patients survived at the last follow-up. Among the eight patients who died, two patients died due to myelodysplastic syndrome, two patients due to leukaemia and two patients due to septic shock. Cerebral infarction and aplastic anaemia were found as the cause of death in the other two patients, respectively. Nine (17.0%) patients had recurrence and three (33.3%) of them eventually died. The time interval from diagnosis to recurrence was between two weeks to 105 months (6.87 ± 18.50 months). Of note, ten cases (18.9%) underwent debridement and three of those cases (30.0%) showed prompt recurrence within one month. Only three patients (5.7%) had complete remission. A comparison between the groups which showed complete remission plus those with partial remission and the group of stable disease plus progressive disease showed that underlying malignancy was more frequently associated in the group of stable disease and progression disease [P = 0.058, Table 3].
| Clinical response to initial treatment, n (%) | P-value‡ | ||||
|---|---|---|---|---|---|
| Complete remission (n = 3) | Partial remission (n = 17) | Stable disease (n = 24) | Progression disease (n = 9) | ||
| Progression free period (median, interquartile range) | CR: 6.00, 4.0–35.0 | PR: 2.0, 1.0–5.0 | SD: 1.0, 0.5–1.0 | PD: 6.0, 3.0–9.0 | |
| CR + PR: 2.5, 1–6 | SD + PD: 1.0, 0.5–3.0 | ||||
| Age | 0.471 | ||||
| Aged ≥ 60 | 1 (33.3%) | 5 (29.4%) | 5 (20.8%) | 2 (22.2%) | |
| Aged < 60 | 2 (66.7%) | 12 (70.6%) | 19 (79.2%) | 7 (77.8%) | |
| Sex | 0.208 | ||||
| Male | 1 (33.3%) | 15 (88.2%) | 14 (58.3%) | 7 (77.8%) | |
| Female | 2 (66.7%) | 2 (11.8%) | 10 (41.7%) | 2 (22.2%) | |
| Mean size of initial lesion (cm) | 2.00 ± 1.00 | 6.21 ± 5.06 | 4.20 ± 2.26 | 6.33 ± 5.85 | 0.501 |
| Duration of skin lesion (month) | 2.00 ± 2.59 | 13.81 ± 24.54 | 6.89 ± 15.07 | 1.33 ± 0.79 | 0.402 |
| Time to diagnosis (month) | 2.00 ± 2.59 | 12.40 ± 21.48 | 5.72 ± 10.56 | 1.33 ± 0.79 | 0.310 |
| Delayed diagnosis | 1 (33.3%) | 4 (23.5%) | 6 (25.0%) | 3 (33.3%) | 0.871 |
| Subtype | |||||
| Ulcerative | 1 (33.3%) | 10 (58.8%) | 13 (54.2%) | 1 (11.1%) | 0.332 |
| Bullous | 1 (33.3%) | 4 (23.5%) | 2 (8.3%) | 5 (55.6%) | 0.623 |
| Vegetative | 1 (33.3%) | 2 (11.8%) | 7 (29.2%) | 1 (11.1%) | 0.399 |
| Pustular | 0 | 0 | 1 (4.2%) | 1 (11.1%) | 0.328 |
| Post-operative | 0 | 1 (5.9%) | 1 (4.2%) | 1 (11.1%) | 0.774 |
| Anatomical location | |||||
| Head and neck | 1 (33.3%) | 3 (17.6%) | 4 (16.7%) | 1 (11.1%) | 0.585 |
| Upper extremities | 1 (33.3%) | 1 (5.9%) | 2 (8.3%) | 0 | 0.456 |
| Trunk | 0 | 1 (5.9%) | 3 (12.5%) | 4 (44.4%) | 0.185 |
| Lower extremities | 1 (33.3%) | 12 (70.6%) | 15 (62.5%) | 4 (44.4%) | 0.772 |
| Multiple lesions | 2 (66.7%) | 6 (35.3%) | 13 (54.2%) | 6 (66.7%) | 0.211 |
| Trauma history | 2 (66.7%) | 4 (23.5%) | 10 (41.7%) | 4 (44.4%) | 0.397 |
| Underlying malignancy | 0 | 5 (29.4%) | 3 (12.5%) | 5 (55.6%) | 0.058† |
When prognosis was correlated with progression-free period, it was significantly shorter in the pyoderma gangrenosum with haematologic disorder group (P = 0.002) and the bullous subtype (P = 0.014) [Figures 3a and 3b]. The pyoderma gangrenosum group in which the size of the initial skin lesion was >4 cm also showed a shorter progression-free period which was however of a borderline significance (P = 0.050) [Figure 3c]. Other clinical variables and histopathologic features did not affect progression-free period [Table 2].
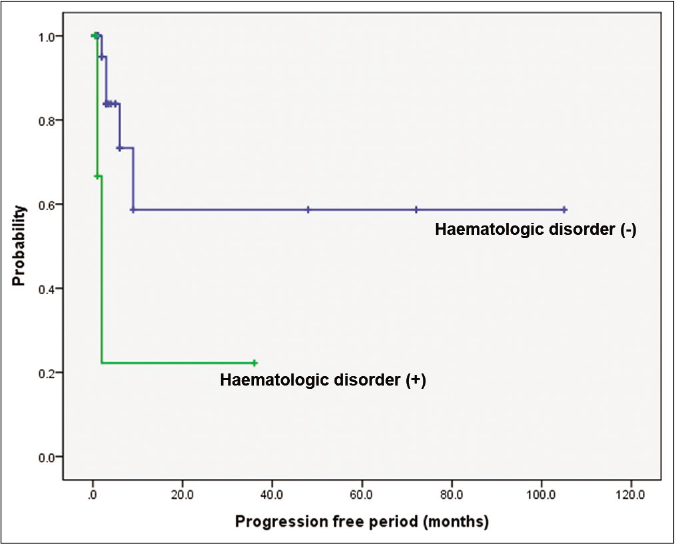
- Comparison of survival according to underlying haematologic disorders and bullous type. Difference in progression-free period depending on underlying haematologic disorders. Worse progression-free period in patients with haematologic disorders (P = 0.002)
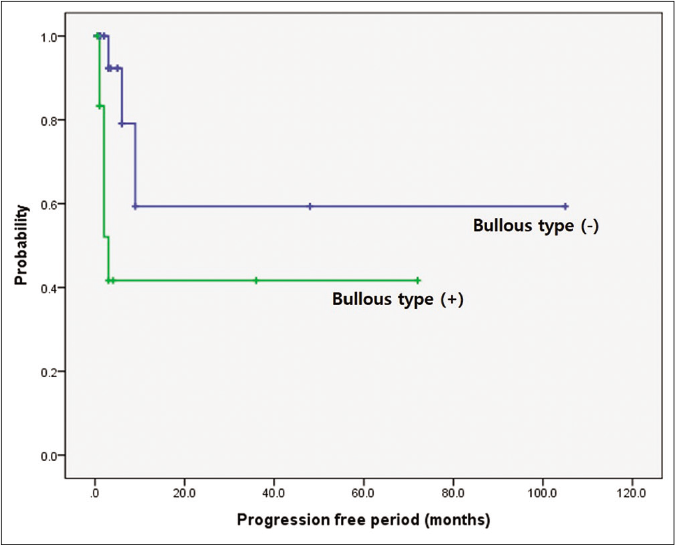
- Comparison of survival according to underlying haematologic disorders and bullous type. Difference in progression-free period depending on bullous subtype. Worse progression-free period in patients with bullous type (P = 0.014)
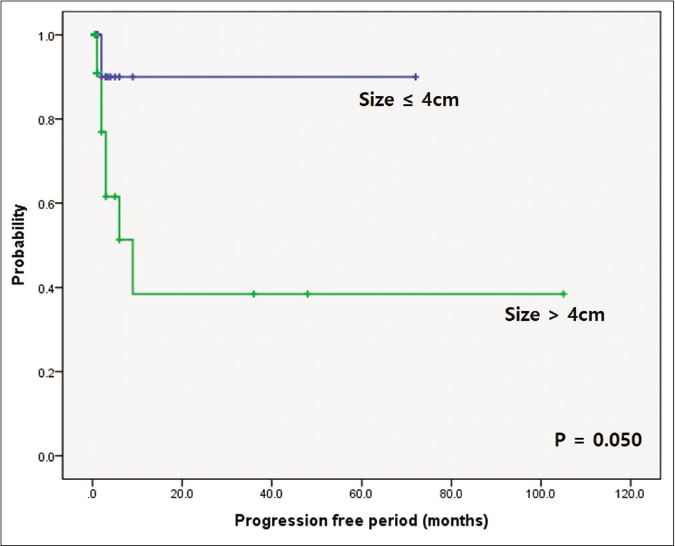
- Comparison of survival according to underlying haematologic disorders and bullous type. Difference in progression-free period depending on initial skin lesion. Worse progression-free period in patients with initial skin lesion over 4 cm (P = 0.050)
Discussion
In our Korean cases, male predominance (M : F ratio = 37/16 vs. 9/27) and younger mean age (43.3 ± 18.5 years vs. 50.9 ± 19.0) were noted as opposed to the Western and Japanese reports.6-8 With regard to pyoderma gangrenosum subtypes, ulcerative type was the most common, which was consistent with previous reports.9 However, ulcerative type (49.1% vs. 78.9%) was less frequent and bullous type (24.5% vs. 1.9%) was more frequent in comparison to the observations in other case series.8,9 Ten cases (18.9%) were associated with inflammatory bowel diseases (Ulcerative colitis and Crohn’s disease), which was less frequent than the incidence rate reported from other countries.10 Brodell et al. found that pyoderma gangrenosum patients of the African– American race had a higher likelihood of development of inflammatory bowel diseases, implying ethnic differences in the clinical manifestations of pyoderma gangrenosum.10
Age-focused workup of patients with pyoderma gangrenosum was suggested by Ashchyan et al.11 as a higher prevalence of solid organ and haematologic malignant disorders was noted in patients aged ≥65 years. They also found a higher prevalence of inflammatory bowel diseases in patients aged <65 years.
However, only five Asians were included among 356 cases in Aschyan’s study. In our study of the Korean population, seven cases aged ≥65 years were observed, and their prognosis was not significantly different than that of others. Patients aged ≥65 years showed no significant association with malignancy (P = 0.343), inflammatory bowel diseases (P = 0.469) or rheumatoid arthritis (P = 0.117) in contrast to Aschyan’s study. Therefore, we concluded that different Asian groups may have ethnical differences and older age does not seem to be a critical prognostic factor for Korean cases at least.
Notably, early gastric cancers were detected in four (7.5%) patients with pyoderma gangrenosum in this study. Gastric cancer is one of the most frequent cancers in the Korean population; however, considering that the incidence of gastric cancer in the general population is only 35.4 cases/100000 persons in the year of 2016,12,13 the higher frequency of gastric cancer in pyoderma gangrenosum is worth remarking upon. Supporting this theory of an increased predisposition to gastric cancer in pyoderma gangrenosum is that it has been reported as the first and isolated clinical manifestation of advanced gastric cancer.14 With regard to pathophysiological considerations, the level of pro-inflammatory cytokines, including tumour necrosis factor α, interleukin-1, interleukin-6 and interleukin-8, were elevated in pyoderma gangrenosum patients,15 and tumour necrosis factor-α inhibitor has been an effective treatment option for pyoderma gangrenosum.16 As the overexpression of tumour necrosis factor-α may lead to an increased gastric cancer risk,17 pyoderma gangrenosum and gastric cancer may be associated through a tumour necrosis factor-α related inflammatory cascade. Conventionally, studies with pyoderma gangrenosum reported other solid tumours, mainly breast cancer, renal caner and colorectal cancer.18-20 A relatively higher incidence of gastric cancer in Korean pyoderma gangrenosum cases is an interesting finding although further investigation is warranted.
A relatively higher frequency of bullous pyoderma gangrenosum in our study is also notable. Bullous pyoderma gangrenosum was found in 13 (24.5%) patients. Only nine cases with bullous pyoderma gangrenosum among 473 cases with pyoderma gangrenosum (1.9%) were observed in a Japanese epidemiologic study.8 Another Japanese regional long-term study also revealed a lower frequency of bullous pyoderma gangrenosum at 6.5%.7 Because up to 70% of bullous pyoderma gangrenosum cases are associated with underlying haematologic disorders,21 clinicians should be cautious when they encounter bullous-type pyoderma gangrenosum. Duguid et al. reported that 75% pyoderma gangrenosum and acute myeloid leukaemia patients died within one year after the manifestation of skin lesions.22 Five cases (38.5%) had haematologic disorders in our study and the presence of haematologic disorders was associated with a frequent recurrence and poor survival rate, consistent with the findings of previous studies.21-24 Due to the association between bullous pyoderma gangrenosum and haematologic disorders, patients with bullous pyoderma gangrenosum should be followed up closely.
Three of 53 cases showed a history of abdominal aortic aneurysm characterised by atherosclerotic changes with chronic inflammation of aortic walls. Two cases with Behcet’s diseases were also observed. The activation of matrix metalloproteinases (MMPs), particularly matrix metalloproteinase-2 and matrix metalloproteinase-9, was the main pathogenesis of both diseases.25 Marzano et al. found that immunoreactivities of matrix metalloproteinase-2 and matrix metalloproteinase-9 were significantly high in pyoderma gangrenosum.26 In light of these findings, although rare, clinicians should be aware of the possibility of underlying fatal aneurysmal diseases, particularly in pyoderma gangrenosum cases.
The histopathologic findings in pyoderma gangrenosum are not specific. Nevertheless, a skin biopsy is recommended in most cases to exclude the other diseases, such as infections and malignancy.3,7,15,21 We initially aimed to find a correlation between the clinical subtype and histopathologic features. Histologic features with granulation tissue were frequently found in post-operative and bullous types. In addition, bullous type pyoderma gangrenosum frequently exhibited granulomas. For these features, the response to initial treatment and the prognosis related to recurrence were analysed for any correlation but no significant difference was found. Since pyoderma gangrenosum has been considered as one of neutrophilic dermatosis,3,27 we also initially thought that the level of neutrophil infiltration may have prognostic significance. However, no significant difference was found between the level of neutrophil infiltration and clinical outcome. Although there are only a few studies on this aspect, we considered it difficult to predict the prognosis of pyoderma gangrenosum based on pathologic features.
The first line of the treatment of pyoderma gangrenosum is an empirical systemic steroid administration because of the lack of controlled studies to date.3,7,15,21 However, delayed diagnosis interferes with the prompt use of systemic steroids. In this study, the use of systemic glucocorticoid or oral immunosuppressant had been chosen for more severe or extensive pyoderma gangrenosum cases, whereas topical agents such as steroid and calcineurin inhibitors had been considered for mild cases with shallow ulcers. In this study, 22 (41.5%) patients with pyoderma gangrenosum were initially treated with a systemic corticosteroid and 15 (68.1%) patients showed partial-to-complete remission. Most of systemic steroid treatment initially taken as prednisolone 1 mg/kg/day and slowly tapered off within two weeks if the treatment response was noticed. Of note, first-line treatment with dapsone showed a remarkable response, as validated in other studies.3,27 In our study, nine (17.0%) of 53 cases experienced recurrence; however, this frequency was lower than the recurrence rates of 24–46% reported in a previous study.7 Because the pyoderma gangrenosum groups with haematologic disorders, bullous type and an initial skin lesion of >4 cm in size showed a significantly short progression-free period, prompt and early administrations of immunosuppressive strategies are recommended in these cases.
The present study had several limitations. This study was a single-centre study with a retrospective design and the sample size was too small to establish a statistically significant association. Due to this, factors such as side effects of each treatment could not be analysed systematically. Moreover, since this is a retrospective study with the clinical data over a period of 20 years, steroid administration such as initial dose and tapering schedules were not uniform. Nevertheless, our study is still noteworthy, because it again proved that pyoderma gangrenosum in haematologic disorders can be recalcitrant and an indicator of poor prognosis. Pyoderma gangrenosum is a still rare cutaneous disease in the Asian population. There are only a few reports dealing with the pattern of disease associations of pyoderma gangrenosum compared to data from Western literature. Our new findings in epidemiologic and clinical aspects with ethnic-specific treatment response data would update the knowledge of clinicians regarding pyoderma gangrenosum.
Conclusion
Pyoderma gangrenosum appeared to have ethnic differences in clinical manifestations such as the frequency of each subtype, associated diseases and prognostic risk factors. Underlying haematologic disorders and bullous subtype have a prognostic significance. The presence of granulation tissue and granuloma was significantly frequent in bullous type; however, histopathologic features did not affect the clinical outcome of pyoderma gangrenosum.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Pyoderma gangrenosum--a review. Orphanet J Rare Dis. 2007;2:19.
- [CrossRef] [PubMed] [Google Scholar]
- Pyoderma (echthyma) gangrenosum: Clinical and experimental observations in five cases occurring in adults. Arch Derm Syphilol. 1930;22:655-80.
- [CrossRef] [Google Scholar]
- Pyoderma gangrenosum: An updated review. J Eur Acad Dermatol Venereol. 2009;23:1008-17.
- [CrossRef] [PubMed] [Google Scholar]
- Etiology and management of pyoderma gangrenosum: A comprehensive review. Am J Clin Dermatol. 2012;13:191-211.
- [CrossRef] [PubMed] [Google Scholar]
- Malignancy grading of the deep invasive margins of oral squamous cell carcinomas has high prognostic value. J Pathol. 1992;166:375-81.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical characteristics and treatment outcomes of 36 pyoderma gangrenosum patients a retrospective, single institution observation. J Eur Acad Dermatol Venereol. 2019;33:e474-5.
- [CrossRef] [PubMed] [Google Scholar]
- Pyoderma gangrenosum and underlying diseases in Japanese patients: A regional long-term study. J Dermatol. 2017;44:1281-4.
- [CrossRef] [PubMed] [Google Scholar]
- Epidemiology of pyoderma gangrenosum in Japanese patients by questionnaire survey. J Dermatol. 2019;46:e145-6.
- [CrossRef] [PubMed] [Google Scholar]
- Skin ulcers misdiagnosed as pyoderma gangrenosum. N Engl J Med. 2002;347:1412-8.
- [CrossRef] [PubMed] [Google Scholar]
- Pyoderma gangrenosum and inflammatory bowel disease: A cross-sectional inpatient socioeconomic study. J Am Acad Dermatol. 2015;73:877-80.
- [CrossRef] [PubMed] [Google Scholar]
- The association of age with clinical presentation and comorbidities of pyoderma gangrenosum. JAMA Dermatol. 2018;154:409-13.
- [CrossRef] [PubMed] [Google Scholar]
- Cancer Statistics. National Cancer Center. Available from: https://www.cancer.go.kr/lay1/S1T639C643/contents.do [Last accessed on 2019 Jul 12]
- [Google Scholar]
- Epidemiology of gastric cancer in Korea. J Korean Med Assoc. 2019;62:398-406.
- [CrossRef] [Google Scholar]
- Pyoderma gangrenosum as first clinical manifestation of gastric adenocarcinoma. J Eur Acad Dermatol Venereol. 2006;20:440-1.
- [CrossRef] [PubMed] [Google Scholar]
- Two cases of postoperative pyoderma gangrenosum. Korean J Dermatol. 2018;56:389-92.
- [Google Scholar]
- Adalimumab treatment for pyoderma gangrenosum. Arch Dermatol. 2007;143:306-8.
- [CrossRef] [PubMed] [Google Scholar]
- The relationship between tumour necrosis factor-alpha polymorphisms and gastric cancer risk: An updated meta-analysis. Biomed Rep. 2017;7:133-42.
- [CrossRef] [PubMed] [Google Scholar]
- Pyoderma gangrenosum associated with solid organ malignancies. Int J Dermatol. 2015;54:e351-7.
- [CrossRef] [PubMed] [Google Scholar]
- Paraneoplastic pyoderma gangrenosum in solid organ malignancy: A literature review. Int J Dermatol. 2020;59:154-8.
- [CrossRef] [PubMed] [Google Scholar]
- Paraneoplastic pyoderma gangrenosum associated with rectal adenocarcinoma. Ann Dermatol. 2018;30:79-82.
- [CrossRef] [PubMed] [Google Scholar]
- Cutaneous indicator of myelodysplastic syndrome: Sudden bullous pyoderma gangrenosum. Jpn J Clin Oncol. 2020;50:958-9.
- [CrossRef] [PubMed] [Google Scholar]
- Bullous pyoderma gangrenosum: A case report and review of the published work. J Dermatol. 2012;39:1010-5.
- [CrossRef] [PubMed] [Google Scholar]
- Pyoderma gangrenosum: An update. Rheum Dis Clin North Am. 2007;33:787-802, vi
- [CrossRef] [PubMed] [Google Scholar]
- Targeted gene disruption of matrix metalloproteinase-9 (gelatinase B) suppresses development of experimental abdominal aortic aneurysms. J Clin Invest. 2000;105:1641-9.
- [CrossRef] [PubMed] [Google Scholar]
- Role of inflammatory cells, cytokines and matrix metalloproteinases in neutrophil-mediated skin diseases. Clin Exp Immunol. 2010;162:100-7.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy of systemic dapsone treatment for pyoderma gangrenosum: A retrospective review. J Drugs Dermatol. 2018;17:1058-60.
- [Google Scholar]






