Translate this page into:
Coexistent cutaneous myeloid sarcoma in a patient with invasive papillary carcinoma of thyroid – A rare presentation
2 Department of Histopathology, Apollo Hospitals, Bhubaneswar, Odisha, India
Correspondence Address:
Satyabrata Tripathy
Department of Dermatology, Apollo Hospitals, Sainik School Road, Bhubaneswar - 751 005, Odisha
India
| How to cite this article: Tripathy S, Mohapatra N, Baisakh M. Coexistent cutaneous myeloid sarcoma in a patient with invasive papillary carcinoma of thyroid – A rare presentation. Indian J Dermatol Venereol Leprol 2018;84:477-481 |
Sir,
A 38-year-old male presented to us with multiple pale red and skin-colored swellings over his scalp, face, trunk and thighs of 1 month duration. Initially, the swellings were asymptomatic, but in the week preceding his visit to our hospital, he developed intense itching over the swellings along with intermittent spikes of high-grade fever with sweating. One month prior to the onset of systemic symptoms, he had undergone a surgical procedure of total thyroidectomy and anterolateral neck dissection for invasive papillary carcinoma of the thyroid. The patient had no family history of any malignancy. The patient could recall the presence of a solitary swelling over his forehead even before the surgery, but since then the swellings had increased in number and size to involve the chest, back, abdomen and thighs. On examination, 52 nodules and tumors were seen scattered over the face, scalp, torso and thighs. They were hard in consistency, immobile, nontender and of varying sizes from 1 cm to 7 cm [Figure - 1]a and [Figure - 1]b. Most nodules were of skin color, baring a few large tumors over the lower back and chest which were erythematous. There was no regional adenopathy or organomegaly. His hepatic, renal and metabolic parameters were all within normal limits at the time of the surgery. His blood count was normal except for a total leukocyte count of 10,200/mm 3 pre-surgically, that went up to 14,200/mm 3 on the first postoperative day and reduced to 12,000/mm 3 on the fourth postoperative day, which was considered a part of normal inflammatory response. No immature cells were seen in the peripheral smear then. However, peripheral blood counts done at presentation showed a total white cell count of 48,000 with 14% blasts.
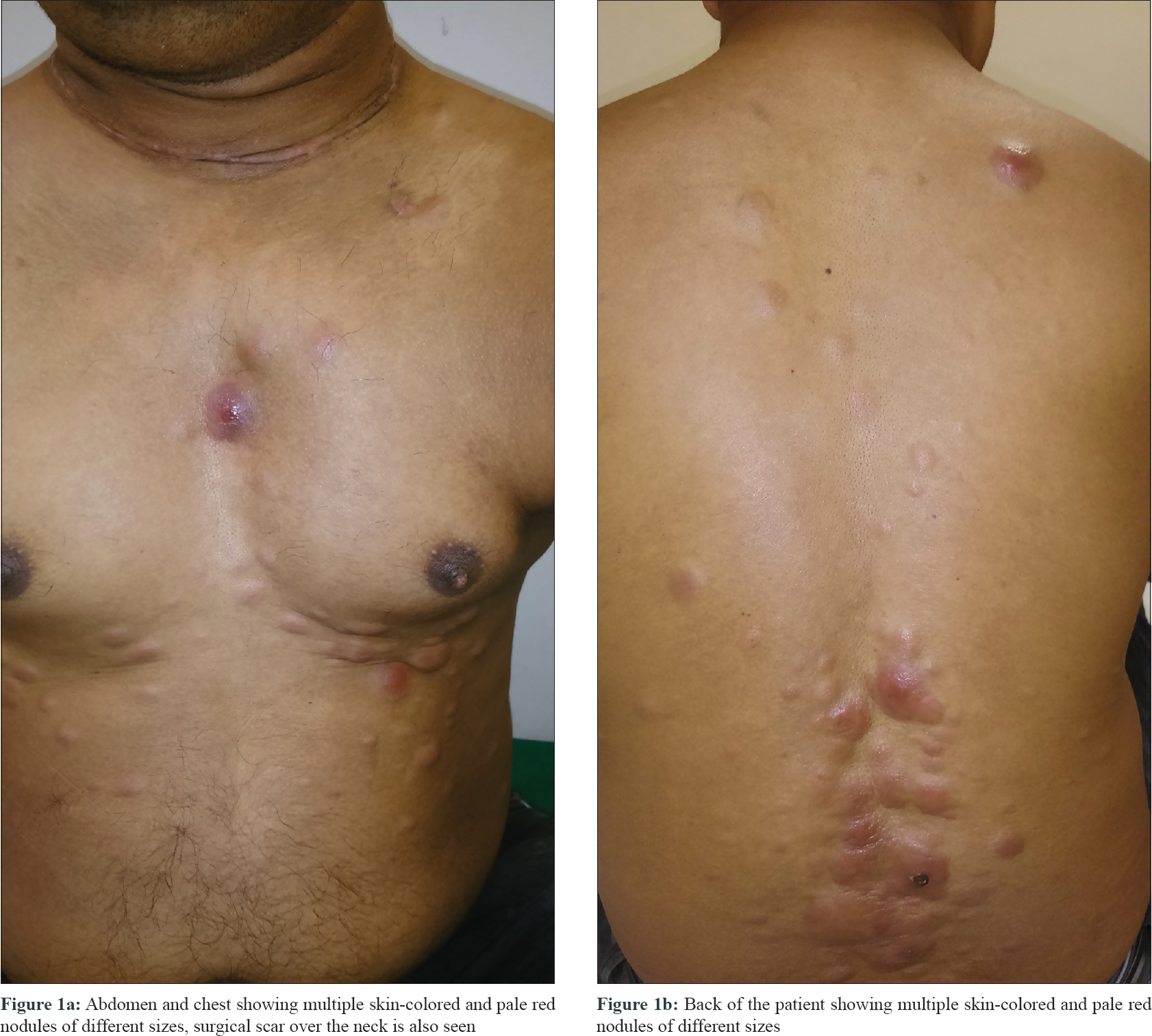 |
| Figure 1: |
The clinical differential diagnoses considered were cutaneous metastatic thyroid nodules, cutaneous lymphoma, sarcoidosis, Sweet's syndrome, nodular amyloidosis or leprosy. A skin biopsy was done for further evaluation. The biopsy showed infiltration by round-to-oval cells in diffuse sheets involving the entire dermis (both superficial and deep dermis) with the destruction of appendages [Figure - 2]a,[Figure - 2]b,[Figure - 2]c. Mitotic figures were scanty. Immunohistochemical investigations revealed that these tumor cells were diffusely and strongly positive for myeloperoxidase [Figure - 3]a, leucocyte common antigen CD117 [Figure - 3]b, CD 45 [Figure - 3]c and CD99 [Figure - 3]d. The Ki 67 labeling index was 95%. Tumor cells were negative for CD3, CD20, CD30, CD56, CD68 and CD34 (markers of myeloid cell line). Based on the myeloperoxidase, CD117 and CD99 positivity, a final diagnosis of cutaneous myeloid sarcoma was made. Bone marrow biopsy revealed the full diagnosis to be acute myeloid leukemia M3 with 28% myeloblasts along with promyelocytes and Auer rods [Figure - 3]e.
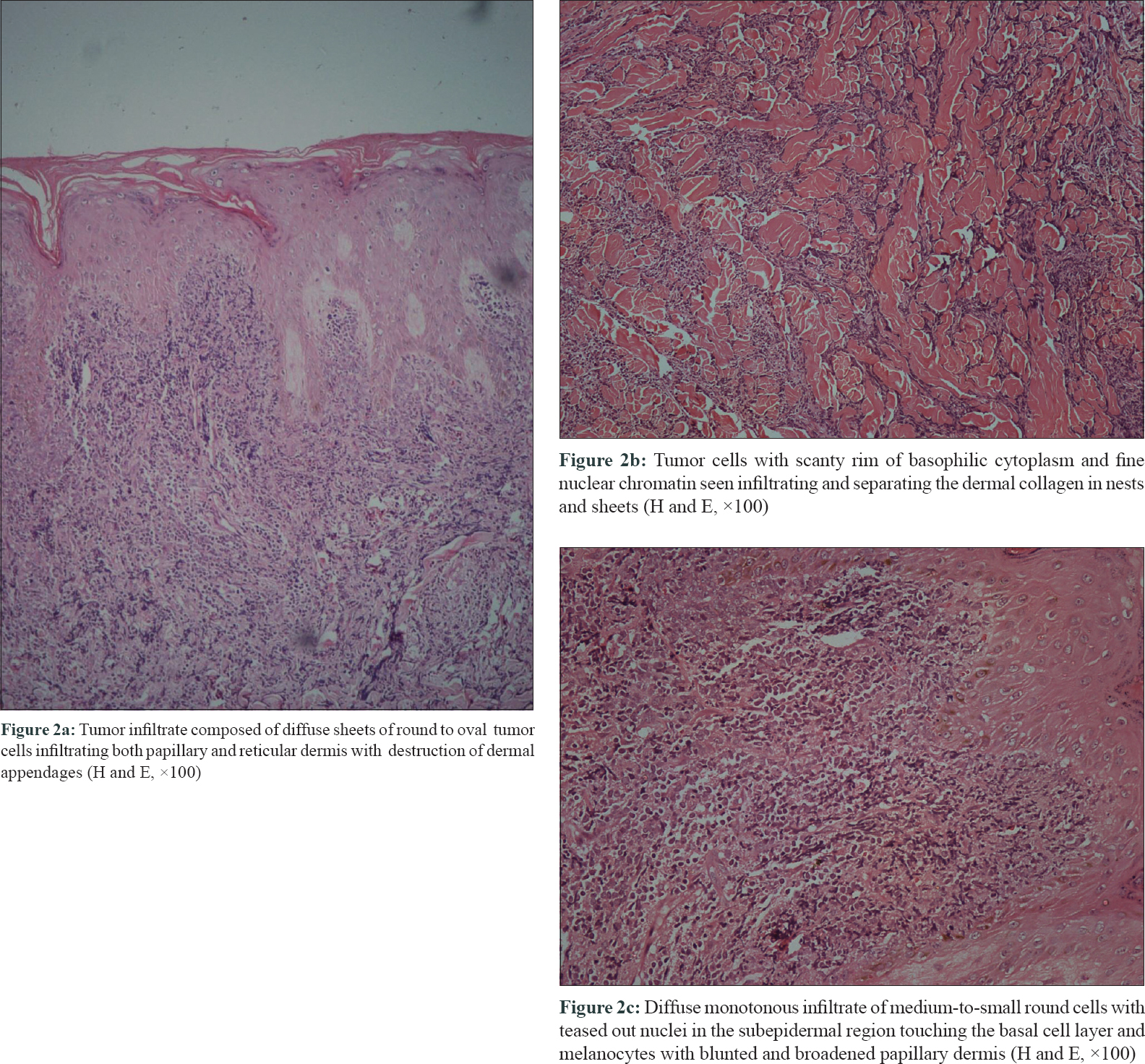 |
| Figure 2: |
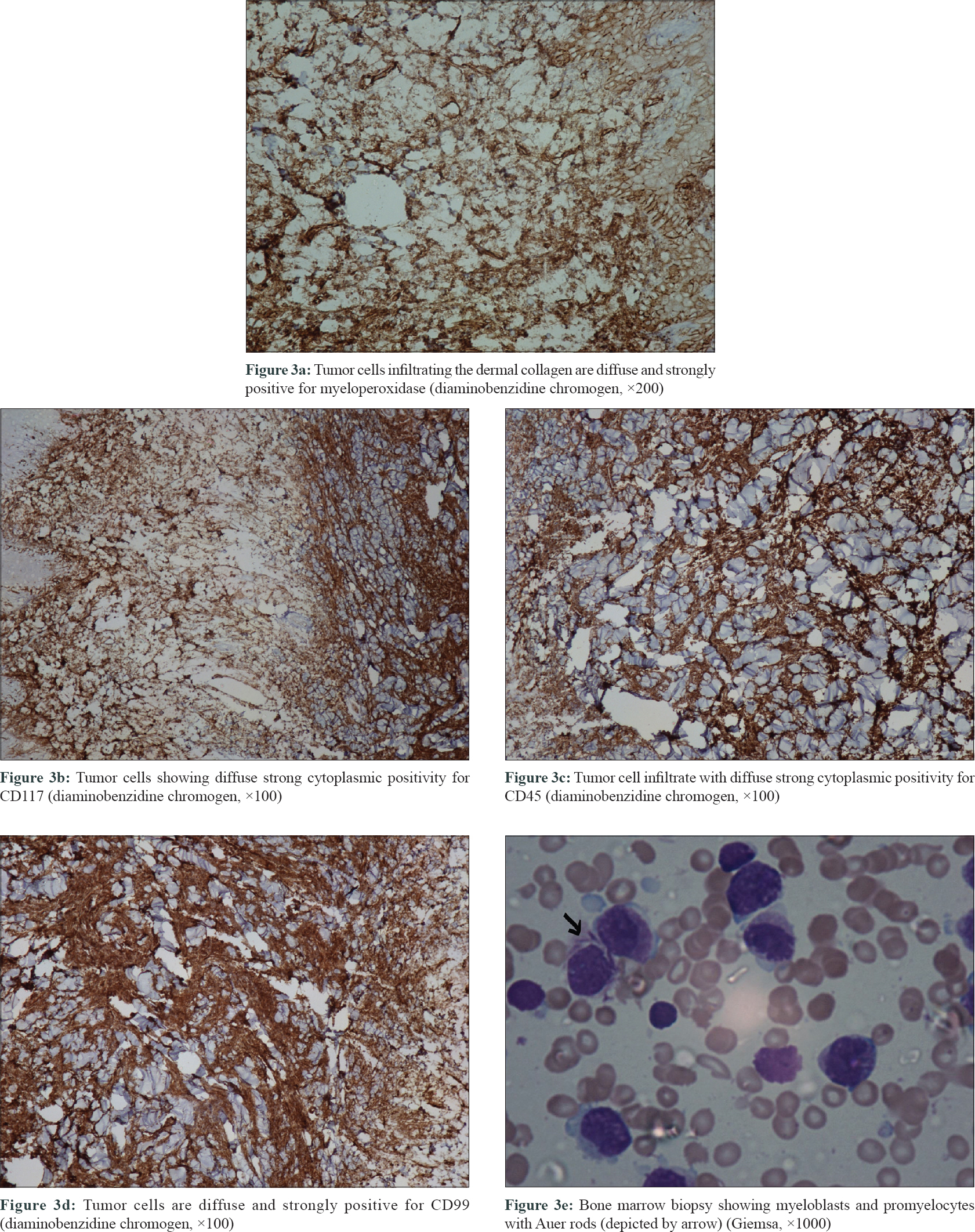 |
| Figure 3 |
As per the World Health Organization, myeloid sarcoma is a tumor mass consisting of myeloid blasts with or without maturation, occurring at a site other than bone marrow.[1] Also known as granulocytic sarcoma or chloroma (as it produces a greenish color due to the presence of enzyme myeloperoxidase), it is an uncommon presentation of underlying myeloid disorders such as acute myeloid leukemia, myeloproliferative neoplasms and myelodysplastic syndromes.2.3 Skin is a common extramedullary site for seeding of such tumor cells, in which case it is termed cutaneous myeloid sarcoma. Other sites reported to be involved are the central nervous system, lymph nodes, gastrointestinal tract, liver, lungs, spleen, oral mucosa and testes.[2],[4] The temporal relationship of cutaneous myeloid sarcoma with underlying bone marrow disease varies; it may precede, follow or occur concomitantly with the diagnosis of leukemia in the marrow. In this case, the cutaneous disease preceded the bone marrow diagnosis.
Myeloid sarcoma is reported in 2.5% to 9.1% of patients with acute myeloid leukemia.[2] Studies of cutaneous involvement in myeloid sarcoma are limited to case reports only, apart from a single 19-year long retrospective study of 83 patients.[4] The presence of cutaneous myeloid sarcoma indicates acute transformation of the underlying bone marrow disease and carries a poor prognosis and high mortality, with most disease-related deaths occurring within the first year of diagnosis.[3],[4] Chromosomal abnormalities, particularly t (8,21), is more frequently associated with it. In the said case, chromosomal studies could not be done due to financial constraints. Further, the family members of the patient were screened for the presence of any malignancy but none were detected.
Leukemia cutis is the infiltration of the epidermis, dermis or subcutis by neoplastic leukocytes (leukemia cells) resulting in clinically identifiable lesions in the form of nodules and plaques. Leukemia cutis as an entity differs from cutaneous myeloid sarcoma in the manner that the cellular infiltrate in the former can be of either myeloid or lymphoid lineage, whereas in the latter condition, the infiltrate comprises mature or immature myeloid blast cells and can involve any extracutaneous site of the body, when it is termed as myeloid sarcoma.
The histopathological differential diagnoses for cutaneous myeloid sarcoma are non-Hodgkin's lymphoma, lymphoblastic leukemia, nodular melanoma, Ewing's sarcoma, blastic plasmacytoid dendritic cell neoplasm and extramedullary haematopoiesis.[2],[4] Immunohistochemistry helps to arrive at a diagnosis using antibodies against sensitive (CD43 and lysozyme) and specific (myeloperoxidase, CD68, CD117 and CD34) markers.2.4 In this case, the cells were myeloperoxidase positive but CD34 negative. Myeloperoxidase and/or CD34 positivity were observed in 56% and 19% of tested cases, respectively, as per the findings of a retrospective study of 83 patients of cutaneous myeloid sarcoma.[4] Myeloperoxidase is the most important marker of myeloid cell line but is positive in only half of the cases. Therefore, a battery of markers are required to establish the myeloid lineage of the cells, which at times might be further complicated by the discordance between the positivity of the cells in bone marrow and skin.[2],[4]
Skin manifestations of internal malignancy are a diagnostic challenge for clinicians. This case is unique in the sense that two unrelated neoplastic conditions coexisted and presented roughly at the same time. Because the patient presented with the skin nodules after surgery for thyroid neoplasm, the onco-surgeon considered it to be metastatic thyroid disease, but biopsy findings suggested an unrelated distinct neoplastic condition. Whether this coexistence is purely a chance association or due to a common genetic alteration remains to be proven. To our knowledge, we could not find any previous reports of cutaneous myeloid sarcoma coexisting with thyroid neoplasm. A report of cutaneous myeloid sarcoma occurring at the site of haematoma and postsurgical scar after surgery for squamous cell carcinoma in the same patient has been described, which has been attributed to Koebners response by the authors.[3] Our patient was referred to a hematology center where he was treated with combination chemotherapy of all-trans retinoic acid with idarubicin and cytarabine as induction regimen followed by 6 mercaptopurine, all-trans retinoic acid and methotrexate as maintenance regimen. The patient successfully completed the induction phase of the therapy, but during the early maintenance phase, he had severe pneumonia and septicemia due to which he succumbed.
To summarize, cutaneous myeloid sarcoma, though uncommon, should always be kept in mind as a differential diagnosis of cutaneous nodules and tumors; biopsy with histopathology and immunohistochemistry study is of utmost importance to arrive at a proper diagnosis. Finally, unrelated neoplastic conditions may coexist in individual patients and pose difficulty and confusion in diagnosis, warranting a multidisciplinary approach for optimal diagnosis and management.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient has given his consent for his images and other clinical information to be reported in the journal. The patient understand that name and initials will not be published and due efforts will be made to conceal identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, et al., editors. WHO Classification of Tumours of Haematopoetic and Lymphoid Tissues. Lyon: IARC Press; 2008.
[Google Scholar]
|
| 2. |
Bakst RL, Tallman MS, Douer D, Yahalom J. How I treat extramedullary acute myeloid leukemia. Blood 2011;118:3785-93.
[Google Scholar]
|
| 3. |
Nizery-Guermeur C, Le Gall-Ianotto C, Brenaut E, Couturier MA, Talagas M, Andrieu-Key S, et al. Cutaneous granulocytic sarcoma and Koebner phenomenon in a context of myelodysplastic syndrome. JAAD Case Rep 2015;1:207-11.
[Google Scholar]
|
| 4. |
Hurley MY, Ghahramani GK, Frisch S, Armbrecht ES, Lind AC, Nguyen TT, et al. Cutaneous myeloid sarcoma: Natural history and biology of an uncommon manifestation of acute myeloid leukemia. Acta Derm Venereol 2013;93:319-24.
[Google Scholar]
|
Fulltext Views
2,304
PDF downloads
2,174





