Translate this page into:
Combination of topical garlic gel and betamethasone valerate cream in the treatment of localized alopecia areata: A double-blind randomized controlled study
2 Department of Pharmacology, Booali Sina Hospital, Mazandaran University of Medical Sciences, Sari, Iran
3 Department of Biostatistics, Booali Sina Hospital, Mazandaran University of Medical Sciences, Sari, Iran
Correspondence Address:
Zohreh Hajheydari
Department of Dermatology, Booali Sina Hospital, Mazandaran University of Medical Sciences, Sari
Iran
| How to cite this article: Hajheydari Z, Jamshidi M, Akbari J, Mohammadpour R. Combination of topical garlic gel and betamethasone valerate cream in the treatment of localized alopecia areata: A double-blind randomized controlled study. Indian J Dermatol Venereol Leprol 2007;73:29-32 |
Abstract
Background: Alopecia areata is a recurrent, nonscarring type of hair loss. Different modalities of treatment have been used to induce hair re-growth. Aims: To determine the efficacy of topical garlic gel in the treatment of alopecia areata. Methods: Patients were randomly divided into two groups of garlic gel and placebo. The two groups were advised to follow the treatment twice daily, for three months. Both groups received topical application of corticosteroid (betamethasone cream 0.1% in isopropyl alcohol) twice daily. Baseline demographic characteristics and the size of patches, total number of grown hair and number of terminal hair at the end of each month were recorded. Effectiveness was assessed by scoring the results. Statistical analysis was done by means of chi-square and t test. Results: Forty patients met the inclusion criteria and enrolled for the study. The first group (garlic treated) consisted of 20 patients (12 males, 60% and eight females, 40%). The second group (control) consisted of 20 patients (10 males, 50% and 10 females, 50%). At the end of the treatment, good and moderate responses were observed in 19 (95%) and one (5%) patients of the case group respectively, which was significantly better than the control group ( P = 0.001). No complication was observed in the patients under study. Conclusion: The present study showed that the use of garlic gel significantly added to the therapeutic efficacy of topical betamethasone valerate in alopecia areata and that it can be an effective adjunctive topical therapy for alopecia areata.


Introduction
Alopecia areata is characterized by round or oval patches of nonscarring hair loss. Men and women are equally affected and the prevalence is almost the same for all ethnic groups.[1],[2],[3] It is a common disease and at any given time, about 0.2% of people are involved with alopecia areata and 1.7% of the populations experience an episode of alopecia areata during their lifetime.[4],[5],[6] The etiology and pathogenesis of alopecia areata is still uncertain, but many factors have been have been described in its pathogenesis, e.g., genetic, family history, the atopic state, nonspecific immune and organ-specific autoimmune reactions, possible emotional stress, infectious agents and neurological factors. A range of treatments have been tried for the treatment of alopecia areata, such as contact sensitizers, immunomodulators and biologic response modifiers.[7],[8],[9],[10],[11] Though different medications with various efficacies have been used for long, no definitive treatment has been introduced yet.[1] Garlic, onion and aromatherapy are claimed to have hair growth promoting properties but the scientific basis is still lacking.[12],[13],[14] Bacteria, fungi, protozoa and viruses have been shown to be sensitive to crushed garlic preparations. Moreover, garlic has been reported to reduce blood lipids and to have anticancer properties. The main active component present in large quantities in garlic cloves is alliin , an oxygenated sulfur amino acid. In crushed garlic, alliin is converted to allicin , which gives the garlic therapeutic properties.[15],[16] Our study is based on anecdotal evidence of garlic therapy that several patients have had marked improvement with this form of therapy. The aim in this study was to test the hypothesis suggesting the presence of pharmacologically active stimulants of hair growth in garlic and that garlic can be a therapeutic agent in patients involved with alopecia areata.
Methods
Study design
This study was a randomized, double-blind, controlled clinical trial of the efficacy of 5% garlic gel in combination with betamethasone cream for three months in patients with alopecia areata. The study was carried out between June 2004 and May 2005 at the Avicenna Hospital, Academic Medical Center of Medicine School, Mazandaran University of Medical Sciences, Iran. The study protocol was approved by the Institutional Ethics Committee and informed written consent was obtained from all the patients under study. The study was conducted in accordance with the guidelines for good clinical practice.
Patients
Patients were from the Iranian male and female population living in southern coastline of the Caspian sea who met the inclusion criteria of the study. Inclusion criteria included patients above five years of age, having up to three hairless patches, overall extension of patches less than 10 cm² (measured using schablone), and duration of disease less than one month. The patients with history of previous treatment, pregnant and lactating women, eyelash and eyebrow involvement, ophiasis pattern and history of sensitivity to garlic were excluded from the study.
Materials
The following chemicals were used as received from the suppliers: methyl and propyl paraben, glycerin (Merk©), HPMC: Hydroxy propyl methyl cellulose K4M (Colorcon©)
Plant material: Allium sativum L . were prepared from Sari, in the North of Iran, in spring 2005. The garlic was blended and extract was prepared by filtration. The fresh extract was used for formulation.
Preparation of the formulations: HPMC was dispersed in preserved water (methyl paraben 0.18% and propyl paraben 0.2%) and glycerin overnight. The extract was added to polymer dispersion and stirred with a double-bladed mixer (IKa-werk©, Germany) 500rpm for 10min and the formulations kept in 4, 25 and 40°C for physical stability evaluation during two weeks. Final formulation for clinical trial was controlled microbiologically based on USP XXIV.
Study procedures
Before enrolment, patients were not receiving treatment with any form of topical or systemic medication. A total of 40 patients were recruited and were randomly assigned into two groups to receive garlic gel 5% or placebo. The two groups were matched for age, sex and stress (using Holmes and Rahe stress test).[17] Garlic gel was rubbed on the alopecia patches under dressing and left for one hour, twice daily for three months in garlic group. The same procedure was carried out in the control group with placebo gel. Both groups received topical corticosteroid (betamethasone cream 0.1% in isopropyl alcohol) twice daily. Clinical evaluation included size of patches (using 1x1 cm² schablone), total number of grown hair and number of terminal hair (count using lens) was performed at the end of each month. The gels of placebo and 5% garlic were prepared in same size and color packages. In order to make the procedure double-blind, the garlic gel was prepared odorless and all of the follow-up evaluations were done by a dermatologist who was blinded to the garlic and control groups.
Scoring of hairlessness was as follows: size (7.6-10 cm²: 1 point, 5.1-7.5 cm²: 2 points, 2.6-5 cm²: 3 points, 0-2.5 cm²: 4 points), number of hair (no hair: 1 point, 1-15: 2 points, 16-30: 3 points, more than 30: 4 points) and number of terminal hair (1-10: 1 point, 11-20: 2 points, 21-30: 3 points, more than 30: 4 points). Treatment outcome was evaluated by giving total score (weak: < 6 points, moderate: 6-9 points, good: ³ 9 points).
Statistical analysis
Statistical analysis of all the qualitative results of this study was done by Chi-square test. Values are expressed as means. The significance of a difference between two groups was calculated using independent t- test with P < 0.05 used as the significant level.
Results
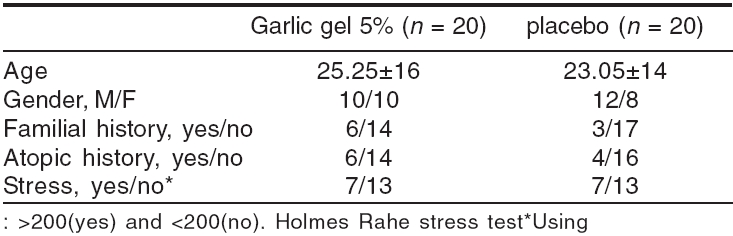
A total of 40 patients entered in this study according to the inclusion criteria and were randomly allocated to either the garlic or control group. Their age ranged from five to 56 years. All of them completed the procedure. Demographic data of all patients under study are given in [Table - 1]. There were no statistically significant differences between these data in the two groups.
The results illustrated in [Table - 2] indicate that in the first month follow-up no statistically significant difference in patch size, number of terminal hairs and number of total hairs in the garlic group has been observed vs. placebo. In the second month, there were significant differences in the number of terminal and total hairs in the two groups ( P = 0.01, P = 0.005). At the end of the third month, the number of total and terminal hairs in the group treated with garlic was significantly ( P = 0.001) higher than those of the control group; and the size of the patches significantly ( P = 0.04) decreased.
Mean of total score in the garlic group at the first, second and third months was 6.5, 8.7, 10.2 respectively, that was significantly ( P < 0.001) more than the baseline score and in the control group mean of total score for each month was 7, 7.5, 8.2 respectively and significant difference was found vs. baseline scores ( P < 0.05).
Pearson′s correlation coefficient between the total number of hairs and total score was 0.80, for the terminal hairs 0.79 and for the size of patches -0.31. At the end of the first month, the therapeutic responses in the garlic group were as follows: no one with good response, moderate in 19 patients and weak in one which indicated no significant difference with the control group. At the end of the second month, good response in 12 patients, moderate in eight and none with weak response; the results were significantly better than those of the control group ( P = 0.001). At the end of the third month, good and moderate responses were respectively observed in 19 and one patients, which was significantly higher than the control group ( P = 0.001). No complication was observed in the patients.
Discussion
In this study, hair growth was observed in all patients. However, the response in the garlic gel group was significantly better than the control group after three months of treatment. Response of the patients to the treatment may be because of corticosteroid application, but the additive efficacy of garlic was demonstrated. In this study, no complications were observed.
Unfortunately, only a few studies have been done about the effectiveness of garlic components in managing alopecia areata. Garlic is used all over the world for different diseases. In Traditional Iranian Medicine (TIM), garlic was prescribed as a remedy for different diseases such as infections, cancers, injuries, gastrointestinal dysfunctions and cardiovascular diseases. Some therapeutic mechanisms of garlic are not clear. The hair growth stimulating mechanisms of garlic are unknown yet. Different researchers have shown that alopecia areata is marked by autoimmune assault on the hair follicle resulting in hair loss.[18] The modulatory effects of garlic on immune responses[19],[20],[21],[22],[23],[24],[25] may justify its efficacy in alopecia areata. In a comparative study by Sharquie et al ., crude onion juice applied topically in treatment of patchy alopecia areata was compared with tap water; it was found that it can be effective in treatment of alopecia areata.[16] Onion and garlic belong to a widely grown vegetable family named Asparagus . Both of them contain diallyl disulfide, which may provide their therapeutic effects.(32) Though different modalities of treatment, local and systemic, have been used to induce hair re-growth, all of them have their own complications and efficacies. The high spontaneous remission rate of alopecia areata, sometimes makes it difficult to clearly assess the true efficacy of a given therapy.[3] In conclusion, this study demonstrates that topical garlic gel treatment may be effective and well tolerated in alopecia areata patients and provides prolonged therapeutic benefits.
| 1. |
Epstein E. Evidence-based treatment of alopecia areata. J Am Acad Dermatol 2001;45:640-2.
[Google Scholar]
|
| 2. |
Sharma VK, Dawn G, Kumar B. Profile of alopecia areata in northern India. Int J Dermatol 1996;35:22-7.
[Google Scholar]
|
| 3. |
Muller SA, Winkelmann RK. Alopecia areata: An evaluation of 736 patients. Arch Dermatol 1963;88:290-7.
[Google Scholar]
|
| 4. |
Firooz A, Firoozabadi MR, Ghazisaidi B, Dowlati Y. Concepts of patients with alopecia areata about their disease. BMC Dermatol 2005;5:1-5.
[Google Scholar]
|
| 5. |
Safavi KH, Muller SA, Suman VJ, Moshell AN, Melton LJ 3rd. Incidence of alopecia areata in Olmsted County, Minnesota, 1975 through 1989. Mayo Clin Proc 1995;70:628-33.
[Google Scholar]
|
| 6. |
Strober BE, Siu K, Alexis AF, Kim G, Washenik K, Sinha A, et al . Etanercept does not effectively treat moderate to severe alopecia areata: An open-label study. J Am Acad Dermatol 2005;52:1082-4.
[Google Scholar]
|
| 7. |
Tang L, Lui H, Sundberg JP, Bissonnette R, McLean DI, Shapiro J. Restoration of hair growth with topical diphencyprone in mouse and rat models of alopecia areata. J Am Acad Dermatol 2003;49:1013-9.
[Google Scholar]
|
| 8. |
Gilhar A, Ullmann Y, Berkutzki T, Assy B, Kalish RS. Autoimmune hair loss (alopecia areata) transferred by T lymphocytes to human scalp explants on SCID mice. J Clin Invest 1998;101:62-7.
[Google Scholar]
|
| 9. |
Shapiro J, Price VH. Hair regrowth. Therapeutic agents. Dermatol Clin 1998;16:341-56.
[Google Scholar]
|
| 10. |
Hoffmann R, Happle R. Topical immunotherapy in alopecia areata: What, how and why? Dermatol Clin 1996;14:739-44.
[Google Scholar]
|
| 11. |
Fiedler VC, Alaiti S. Treatment of alopecia areata. Dermatol Clin 1996;14:733-7.
[Google Scholar]
|
| 12. |
Sharquie KE, Al-Obaidi HK. Onion juice (Allium cepa L.), a new topical treatment for alopecia areata. J Dermatol 2002;29:343-6.
[Google Scholar]
|
| 13. |
Hay IC, Jamieson M, Ormerod AD. Randomized trial of aromatherapy. Successful treatment for alopecia areata. Arch Dermatol 1998;134:1349-52.
[Google Scholar]
|
| 14. |
Lee TY, Lam TH. Contact dermatitis due to topical treatment with garlic in Hong Kong. Contact Dermat 1991;24:193-6.
[Google Scholar]
|
| 15. |
Ankri S, Mirelman D. Antimicrobial properties of allicin from garlic. Microbes Infect 1999;1:125-9.
[Google Scholar]
|
| 16. |
Arnault I, Haffner T, Siess MH, Vollmar A, Kahane R, Auger J. Analytical method for appreciation of garlic therapeutic potential and for validation of a new formulation. J Pharm Biomed Anal 2005;37:963-70.
[Google Scholar]
|
| 17. |
Holmes TH, Rahe RH. The social readjustment rating scale. J Psychosom Res 1967;11:213-8.
[Google Scholar]
|
| 18. |
Micali G, Cicero RL, Nasca MR, Sapuppo A. Treatment of alopecia areata with squaric acid dibutylester. Int J Dermatol 1996;35:52-6.
[Google Scholar]
|
| 19. |
Papageorgiou C, Corbet JP, Menezes-Brandao F, Pecegueiro M, Benezra C. Allergic contact dermatitis to garlic ( Allium sativum L.). Identification of the allergens: the role of mono-, di-, and trisulfides present in garlic. A comparative study in man and animal (guinea-pig). Arch Dermatol Res 1983;275:229-34.
[Google Scholar]
|
| 20. |
Delaney TA, Donnelly AM. Garlic dermatitis. Australas J Dermatol 1996;37:109-10.
[Google Scholar]
|
| 21. |
Lau BH, Yamasaki T, Gridley DS. Garlic compounds modulate macrophage and T-lymphocyte functions. Mol Biother 1991;3:103-7.
[Google Scholar]
|
| 22. |
Morioka N, Sze LL, Moron DL, Irie RF. A protein fraction from aged garlic extract enhances cytotoxicity and proliferation of human lymphocytes mediated by interleukin-2 and concanavalin A. Cancer Immunol Immunother 1993;37:316-22.
[Google Scholar]
|
| 23. |
Jeong HG, Lee YW. Protective effects of diallyl sulfide on nitrosodimethylamine-Induced immunosuppression in mice. Cancer Lett 1998;134:73-9.
[Google Scholar]
|
| 24. |
Tang Z, Sheng Z, Liu S, Jian X, Sun K, Yan M. The preventing function of garlic on experimental oral precancer and its effect on natural killer cells, T-lymphocytes and interleukin-2. Hunan Yi Ke Da Xue Xue Bao 1997;22:246-8.
[Google Scholar]
|
| 25. |
Tabar AI, Alvarez MJ, Celay E, Lopez R, de Esteban B, Gomez B. Allergy to asparagus. An Sist Sanit Navar 2003;26:17-23.
[Google Scholar]
|
Fulltext Views
7,689
PDF downloads
2,940





