Translate this page into:
Comorbidities in psoriasis
Correspondence Address:
Sanjeev J Aurangabadkar
Skin and Laser Clinic, 1st Floor, 'Brij Tarang' Green Lands, Begumpet, Hyderabad
India
| How to cite this article: Aurangabadkar SJ. Comorbidities in psoriasis. Indian J Dermatol Venereol Leprol 2013;79:10-17 |
Abstract
Moderate to severe psoriasis is associated with concomitant diseases that may have a significant impact on patients. It is necessary for the treating physician to recognize these concomitant diseases, known as comorbidities, early as they influence the management options. Important comorbidities are psoriatic arthritis, metabolic syndrome, Crohn's disease, depression, and cancer. Patients with severe psoriasis may be at an increased risk for myocardial infarction and this subgroup of patients tends to have a reduced life expectancy. The presence of co-morbid diseases is associated with an increase in concomitant medication, some of which may worsen psoriasis; conversely, systemic treatment of psoriasis with certain drugs may impact the co-morbid conditions. As dermatologists are the primary health-care providers for psoriasis, adequate knowledge of comorbidities helps in choosing the appropriate therapy as well as timely intervention.Introduction
Psoriasis is a multi-system inflammatory disease where the skin and the joints are the primary targets. There are many reports that psoriatic patients tend to have concurrent illnesses that are termed as comorbidities, though there are remarkably few studies from India. The choice of therapy of psoriasis may be influenced by their coexistence, and the systemic treatment of psoriasis with certain drugs may impact them negatively. Dermatologists should be aware of these associations as they may be in a position to detect them early, thus, allowing early intervention that may improve the overall quality of life of the patient.
Comorbidities in Psoriasis
Comorbidities can be classified as physical and psychosocial [Table - 1] and [Figure - 1]. Onumah et al. observed that the severity of psoriatic skin disease portends a serious risk for the development of these comorbidities; [1] patients with moderate to severe psoriatic skin disease have a higher association with these comorbidities, [1] which may be related through common pathogenic mechanisms.
Comorbidities may increase with age; one recent study found that patients older than 65 years had a statistically significant higher prevalence of hypertension, left ventricular hypertrophy, waist-hip ratio, diabetes mellitus and raised blood glucose levels. Psoriatic patients had a 4-fold increased risk of type 2 diabetes, 3-fold risk of myocardial infarction and life expectancy shortened by 4 years compared to healthy controls. [2] Nearly half of all the psoriasis patients above 65 years of age have at least three co-morbidities. [2] In one study, patients with severe psoriasis were found to die about 3-4 years earlier than patients without psoriasis. [3]
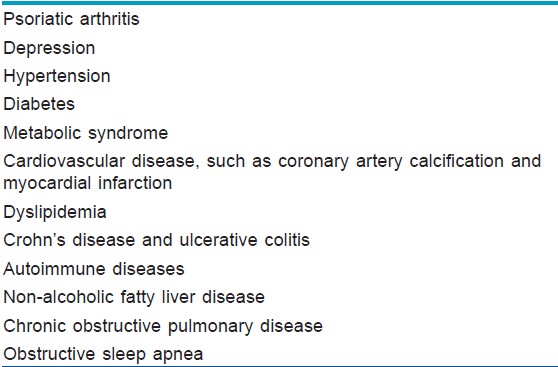
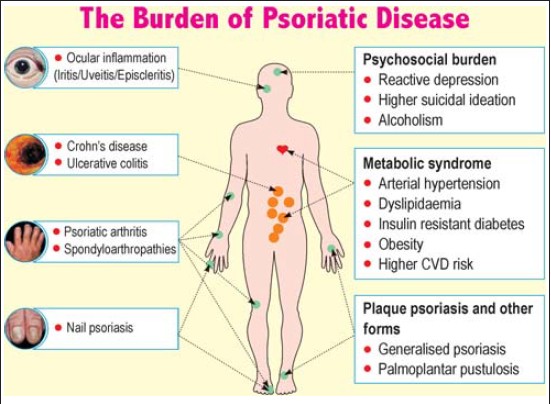 |
| Figure 1: Comorbidities associated with psoriasis |
Pathogenesis of psoriasis and comorbidities
While the numerous susceptibility loci for psoriasis and psoriatic arthritis (PsA) explains only a part of the heritability of the disease, newer models of psoriatic pathogenesis combine skin barrier function, T-helper 17 (Th17) pathway, innate immunity, signaling pathways, Th2 pathway, and adaptive immunity involving CD8 T cells. These studies illustrate the importance of both the keratinocytes and the immune system for the pathophysiology of psoriasis.
Additional studies investigating the role of psoriasis activity and severity as an independent risk factor for developing metabolic disorders, atherosclerosis and myocardial infarction and the role of psoriasis treatment in altering the risk of developing these serious morbidities are urgently needed. [4]
Common inflammatory pathways and genetic predispositions for specific patterns in the immune response may play a vital role in the evolution of associated conditions, e.g., human leucocyte antigen HLA Cw6 in psoriasis and PsA, tumor necrosis factor alpha (TNF-α) in psoriasis and PsA, and interleukin (IL)-12/23 in psoriasis and Crohn′s disease (CD). [4] Azfar and Gelfand found that the psoriasis susceptibility loci psoriasis susceptibility genes PSORS2, PSORS3, and PSORS4 are also associated with loci of susceptibility for metabolic syndrome, type 2 diabetes, familial hyperlipidemia, and cardiovascular (CV) disease. [4] Several regions on chromosomes 16, 6, 4, and 3 have been identified where genetic markers are linked to both psoriasis and CD. [4] The susceptibility loci of psoriasis, CD, and ulcerative colitis (UC) appear in the 6P21 locus, an area that encompasses the utmost histocompatibility complex. [4] This area includes the inflammatory bowel disease 3 (IBD3) locus involved in CD and UC and the PSORS1 locus involved in psoriasis. Moreover, several non-major histocompatibility complex-related genes, such as the IL-23 receptor and IL-12B genes, have also been associated with psoriasis, CD, and UC. [4]
In addition to a genetic correlation, common inflammatory pathways exist for psoriasis and IBD. Psoriasis and CD are mediated by T-helper 1 (Th1) lymphocytes producing cytokines such as TNF-α and interferon (IFN)-γ. Th 17 cells also play a vital role in psoriasis and CD, producing IL-17, IFN-γ, and IL-21 cytokines. Increased levels of IL-17 and IL-23 in the intestinal lamina propria of patients with CD, in the serum, and in the cutaneous lesions of psoriatic patients have been noted. [5]
Psychosocial Comorbidities
Psoriasis is associated with a variety of psychological problems, including poor self-esteem, sexual dysfunction, anxiety, depression, and suicidal ideation, reported as high as 67% in one study. [6] This is not surprising since psychosocial comorbidities are more likely to occur in any chronic disease, especially the one which is visible, and with the attendant problems of smoking and alcohol abuse. These psychosocial co-morbidities are not always proportional to or predicted by, other measurements of disease severity such as body surface area involvement or plaque severity. [7] It has been suggested that it is essential to include measures of psychosocial morbidity when assessing psoriasis severity and treatment efficacy because of the substantial role that psychosocial burden plays in patient′s perception of disease severity, quality of life, and disease course. [8]
Multiple studies have concluded that psoriasis sufferers feel self-conscious, disturbed or inconvenienced by the shedding of the skin, live in a constant fear of relapse, and avoid social interactions. [9],[10] In one study, the disease affected their social functioning and led to decreased efficiency and subjective distress at work in more than half of the subjects. [6] Those who develop psoriasis at a younger age may have stronger feelings of stigmatization than those with a similar clinical picture later in life. [11]
Kimball et al. found that patients with psoriasis were significantly more at risk of developing psychiatric disorders versus control subjects (5.13% vs. 4.07), especially depression (3.01% vs. 2.42) and anxiety (1.81% vs. 1.35%). [12] Other studies have demonstrated up to a 45% prevalence of anxiety among psoriasis patients, more commonly in women. [13] One study also found that psoriatic women were more depressed than psoriatic men. [14] Depression and suicidal ideation are relatively more common in patients with extensive psoriatic disease than in milder ones. [15],[16]
Kremers et al. noted that patients with psoriasis have a higher prevalence of smoking and alcohol consumption. [17] Psoriasis appears to be exacerbated by drinking habit. [18] The amount of alcohol consumption may be related to both a higher incidence and severity of psoriasis. The mechanisms by which alcohol affects psoriasis include the production of pro-inflammatory cytokines, stimulation of lymphocyte and keratinocyte proliferation, and increased susceptibility to infections. TNF alpha has been found to play a crucial role in alcoholic hepatitis. Alcohol misuse can predispose to an increased risk of liver disease and drug interactions. Alcoholic and non-alcoholic liver diseases have both found to be common in patients of psoriasis. [18]
Thus, psoriasis leads to significant psychosocial disability and psychological comorbidities lead to poor treatment outcomes and worsening or precipitation of disease. [19] This facet of the disease should be addressed by psychological and behavior therapy. [20]
Psoriatic Arthritis (PsA)
PsA is the most common association. It has been discussed separately in another article.
Metabolic Syndrome
Psoriasis is associated with metabolic syndrome, which encompasses obesity, raised triglycerides, low high density lipoprotein (HDL), insulin resistance, and hypertension. Its importance lies in its ability to predispose sufferers to CV disease. There are some reports that the association is stronger for severe psoriasis than for mild psoriasis, [21],[22] but this association has still not been established beyond doubt as the number of studies are few and some studies have conflicting results.
The updated Adult Treatment Panel III (ATPIII) criteria for the diagnosis of metabolic syndrome includes three or more of the following: triglyceride ≥ 150 mg/dl (1.7 mmol/l), HDL cholesterol < 40 mg/dl (1.03 mmol/l) in men and < 50 mg/dl (1.29 mmol/l) in women, fasting glucose ≥ 100 mg/dl (5.6 mmol/l) or previously diagnosed with type 2 diabetes, blood pressure ≥ 130/85 mmHg or on antihypertensive medication, and central obesity (defined as waist circumference ≥ 90 cm in men and ≥ 80 cm in women, according to the ethnic criteria for Asians). The International Diabetes federation (IDF) requires central obesity (defined as waist circumference ≥ 90 cm in men and ≥ 80 cm in women for Asians, except for Japanese) plus two of the following four factors: triglycerides ≥ 150 mg/dl, HDL cholesterol < 40 mg/dl in men and < 50 mg/dl in women, fasting glucose ≥ 100 mg/dl or previously diagnosed with type 2 diabetes, and blood pressure ≥ 130/85 mmHg or on treatment for hypertension. [23],[24]
The most common feature of the metabolic syndrome among patients with psoriasis was abdominal obesity, followed by hypertriglyceridemia and low levels of HDL cholesterol. [3] The prevalence was higher in women than in men with psoriasis. Obesity itself is an independent risk factor for developing psoriasis. Sterry et al. found that obese patients were more likely to have severe psoriasis (i.e. >20% body surface area). Intra-abdominal obesity was directly linked to the metabolic syndrome. [25] Several studies have shown that psoriasis is associated with atherogenic dyslipidemia with increased blood levels of total cholesterol, triglycerides, low density lipoprotein (LDL), very low density lipoprotein, and lipoprotein A, and low levels of HDL and apolipoprotein B. [26]
Women with psoriasis showed a 63% increased risk of future diabetes compared with women without psoriasis. [3] In addition to a relationship between psoriasis and elevated blood glucose and blood pressure in the context of metabolic syndrome, observational studies have detected independent associations between psoriasis and hypertension and diabetes.
The pathophysiology of metabolic syndrome is attributed to insulin resistance mediated by adipocytokines, such as TNF-α, leptin, and adiponectin. The predominant underlying risk factor is visceral adiposity. [27] Elevated levels of leptin and its receptor are found in the serum and skin of psoriasis patients, which is thought to result from adipose stores as well as inflammatory processes. [28] Adiponectin is a circulating hormone produced by adipocytes that has antiinflammatory activity by suppressing production of TNF-α, IL-6, and INF-γ, improves insulin sensitivity and has antiatherogenic effects. [28] Leptin, produced by adipocytes, participates in immune and inflammatory processes, stimulating the release of pro-inflammatory cytokines. Adiponectin exerts an opposing action promoting sensitization to insulin, reduction of TNF-α production and macrophage phagocytic activity. Obesity, mainly visceral causes hypoadiponectinemia, which results in higher CV risk. [29] Naldi et al. found that leptin deficiency negates autoimmune pathophysiology, suggesting a potential link between adipose tissue and psoriatic inflammation. [30]
A state of chronic low grade inflammation exists in obese patients and this could explain the role that obesity plays in the development of psoriasis and a new report showed a direct correlation between obesity as measured according to different parameters and psoriasis severity. [31] A high level of TNF-α, IL-6 and C-reactive protein is found in patients with high body mass index. This, in turn, may lead to alterations in insulin sensitivity/resistance and higher oxidative stress and the production of free radicals. These pro-inflammatory cytokines could affect the course and presentation of psoriasis. Food containing free fatty acids may activate toll-like receptors 4 on the cellular surface of adipocytes and macrophages, thereby producing a state of insulin resistance.
It is likely that a state of chronic low-grade inflammation and traditional risk factors both contribute to the predisposition of psoriasis patients to CV and metabolic comorbidities. Boehncke et al. [32] have proposed the concept of the ′psoriatic march′, which while not formally proven, suggests a causal link between psoriasis as a systemic inflammatory condition and CV comorbidity. According to this hypothesis, systemic inflammation may cause insulin resistance, which triggers endothelial cell dysfunction, subsequently leading to atherosclerosis and finally myocardial infarction or stroke. [32]
CV Disease and Psoriasis
Data support the association of psoriasis with CV risk, especially in cases of severe psoriasis, where patients are on long term systemic drugs and/or have been hospitalized for psoriasis. The higher prevalence of CV risk factors may predispose to an increased risk of atherosclerosis as well as myocardial infarction. Psoriasis confers an independent risk for myocardial infarction, the relative risk being greatest in young patients with severe psoriasis.
CV conditions significantly associated with psoriasis are ischemic heart disease, angina and myocardial infarction; cerebrovascular disease, transient ischemic attack, and stroke; and peripheral vascular diseases. CV conditions sporadically associated with psoriasis are arrhythmia, structural heart disorders, and pulmonary hypertension. Subclinical CV changes significantly associated with psoriasis are coronary artery calcification, arterial stiffness, and increase in intima-media thickness. [33]
Psoriasis might be associated with endothelial dysfunction, both because of the abundance of pro-inflammatory cytokines as well as the metabolic abnormalities found in it. [34] Th1/Th2 imbalances are involved in the pathogenesis of both atherosclerosis and psoriasis. [6] The proinflammatory cytokines IFN-γ, TNF-α, IL-1, and IL-6, may favor the development of atherosclerosis and ultimately acute coronary syndrome as well as psoriasis. [33] Osteopontin, an inflammatory glycoprotein that exerts a Th1 cytokine effect is thought to play a role in the development of atherosclerosis. Psoriasis is an independent risk factor for elevated osteopontin levels. [28]
Use of TNF-α inhibitors for psoriasis was associated with a significant reduction in the myocardial infarction risk and incident rate compared with treatment with topical agents. [35] The study also found that the use of TNF-α inhibitors for psoriasis was associated with a non-statistically significant lower myocardial infarction incident rate compared to treatment with oral agents or phototherapy. [35]
Psoriasis and Liver and Gastrointestinal Tract
Non-alcoholic fatty liver disease (NAFLD) is more prevalent in obese patients without exposure to an obvious cause. It includes a spectrum of conditions ranging from simple fatty liver to non-alcoholic steatohepatitis (NASH), which can give rise to fibrosis, cirrhosis, and eventually hepatocarcinoma. NAFLD is now regarded as the hepatic manifestation of the metabolic syndrome. It also leads to endothelial dysfunction, thus leading to CV disease.
In a recent cross-sectional study, the frequency of NAFLD in patients with psoriasis was found remarkably greater than in controls (47% vs. 28%). [5] Psoriatic patients with NAFLD were more likely to have the metabolic syndrome and had significantly higher serum C-reactive protein (CRP) and IL-6 levels and lower serum adiponectin levels than those with psoriasis alone. Patients with psoriasis and NAFLD (and specifically, non-alcoholic steatohepatitis - NASH) are at an increased risk for methotrexate (MTX)-induced hepatotoxicity. [5]
In an Indian study, the occurrence of NAFLD was higher in psoriasis patients than in controls (17.4 vs.7.9%; P = 0.002). NAFLD patients in the psoriasis group were more likely to have metabolic syndrome and diabetes than those with psoriasis alone. The former group had a longer duration of psoriasis and arthritis. Psoriasis patients with NAFLD had more severe disease. Psoriasis patients had more severe NAFLD than controls as reflected by the steatosis, NASH and fibrosis scores. The authors concluded that NAFLD is the commonest liver disease in Indian psoriatic patients when compared to controls. [36]
Binus et al. found that patients with both psoriasis and inflammatory bowel disease (IBD) had significantly higher rates of autoimmune thyroiditis, hepatitis and diabetes as well as seronegative arthritis. [37] They concluded that patients with both psoriasis and IBD have a number of further associated comorbidities, some at significantly higher levels than in individuals with psoriasis only. Psoriasis and CD are inflammatory disorders primarily mediated by Th1 lymphocytes producing cytokines such as TNF-α and IFN-γ. Family members of patients with psoriasis and Crohn′s disease have a higher incidence of the other disease. [37],[38] In a recent study, the prevalence of celiac disease in patients with psoriasis was significantly higher than in controls (0.29% vs. 0.11%). [5] Ludvigsson et al. in a Swedish cohort study of 401 patients concluded that individuals with celiac disease were at increased risk of psoriasis both before and after celiac disease diagnosis. [39]
Psoriasis and Cancer
The cancer risk is elevated in severe psoriasis cases. [40] Patients who have used systemic therapies have a higher incidence of non-melanoma skin cancers and lymphoproliferative diseases. [40] Systemic PUVA treated patients too are at an increased risk of non-melanoma skin cancers, and this risk remains increased up to 15 years after stopping PUVA. Bath PUVA is not associated with this risk. Psoriatics with mild disease also have a slightly higher incidence of cancer. [41]
Psoriasis patients over 65 years have a threefold increase in lymphomas. [40] Other cancers are also more prevalent in psoriasis: carcinoma of the oral cavity and pharynx, esophagus, liver, pancreas, lung, skin (squamous cell carcinoma), bladder, kidney, female breast, male genital cancers, and mycosis fungoides in men. [41]
Frentz and Olsen found psoriatics to have an elevated risk of squamous cell carcinoma (observed/expected ratio - O/E: 4.1), basal cell carcinoma (O/E: 2.2), cancer of the lung (O/E: 1.5), oral cavity (O/E: 2.3), larynx (O/E: 2.4), and pharynx (O/E: 4.1) in men; and cancer of the lung (O/E:1.6), colon (O/E: 1.4) and "unspecified sites" (O/E: 2.5) in women. It is unclear if the increased risk of malignancy is secondary to the potentially carcinogenic and immunosuppressive agents used to treat psoriasis or due to smoking and alcoholism which is more common in psoriatics or some genetic predisposition. [42]
Psoriasis and Chronic Obstructive Pulmonary Disease (COPD)
Increased rates of chronic obstructive pulmonary disease (COPD) have been detected in patients with psoriasis. A Taiwanese study observed that psoriasis patients were at a greater risk of developing COPD with significantly lower COPD-free survival rates than the comparison cohort. [43] Another large, population-based case-control study found that the prevalence of COPD was significantly higher in patients with psoriasis (5.7% vs. 3.6%, P < 0.001, odds ratio [OR] 1.63). The authors recommended that dermatologists caring for patients with psoriasis should be aware of this association, consult a physician or a pulmonologist, and advise patients to stop smoking and reduce additional risk factors for COPD. [44]
Psoriasis and the Eye
Eye inflammation, especially uveitis is a prominent feature of spondyloarthropathies. Uveitis associated with undifferentiated spondyloarthropathy, inflammatory bowel disease, and psoriasis may be less characteristic in its presentation, with a higher tendency of the posterior pole involvement, bilaterality, and chronicity. [45] Lima et al. found keratoconjunctivitis sicca to be the most common ocular finding related to PsA. [46]
Longevity
Psoriatic patients may have decreased longevity. Riech reported that co-morbidities are likely to contribute to the 3- to 4-year reduction in life expectancy, in patients of severe psoriasis. [47] The mortality may be related to the age of onset of psoriasis; the decrease in longevity may be as much as 20 years in patients whose psoriasis begins before 25 years of age.
Comorbidities and Management of Psoriasis
Comorbidities also mean more medications for patients, adding to the financial burden to the patient and the health care system. In one study, patients with psoriasis had higher prescription rates for all drugs associated with the metabolic syndrome (with an absolute maximum difference of 5%) compared with the reference population, but they were also more likely to have used other prescription drugs. [48] They used significantly more antihypertensives, anticoagulant and antiplatelet agents, digoxin, nitrates, lipid lowering and antidiabetic drugs than the reference population during a 5-year period observation. [48]
Drugs that can trigger psoriasis include beta blockers, lithium, antimalarials, and angiotensin converting enzyme (ACE) inhibitors. ACE inhibitors and beta blockers are used for hypertension, which is a well-known comorbidity of psoriasis and part of the metabolic syndrome. [49]
Treatment options must take into consideration the patients′ comorbidities (to identify contraindications) and co-medications (to avoid drug interactions). Comorbidities not only reduce therapeutic options, but can potentially be aggravated by anti-psoriatic treatment. Acitretin may increase triglycerides and cholesterol, negatively influencing the dyslipoproteinemia linked to metabolic syndrome.
In some patients, cyclosporin can cause or worsen hypertension and may also increase serum lipids, thus increasing the risk of endothelial dysfunction and CVD. [50] At the same time, it may reduce the risk of CV disease in psoriatics by decreasing inflammation.
Methotrexate (MTX), one of the most frequently used antipsoriasis drugs has to be used with caution in the presence of diabetes, obesity, and alcohol consumption. Riech suggested that successful treatment with MTX appears to lower the rates of myocardial infarction (MI) in patients with psoriasis. [47]
Natural sunlight and UV phototherapy, effective in the treatment of psoriasis, can potentially induce a degree of systemic immunosuppression mediated via Th2 cytokines IL-4 and IL-10. UV-induced vitamin D production also reduces the risk for atherosclerosis by augmentation of IL-10 and down-regulation of TNF-α, C-reactive protein (CRP) and IL-6 production and modulation of the renin-angiotensin system. [51]
In addition to their lipid-lowering effects, statins have potential immunosuppressive benefits by reducing the inflammatory load and risk of CV diseases in patients with elevated cholesterol levels, thus validating their benefit in psoriasis. [52] Statins reduce the annual incidence of stroke by approximately 30% in patients with coronary artery disease, an effect that may be due to anti-inflammatory actions in addition to lowering cholesterol levels. The anti-inflammatory effects may be due to inhibition of the inflammatory activity of macrophages or a reduction of tissue factor and matrix metalloproteinase (MMP) expression. In addition, statins have down-regulatory effects on systemic markers of inflammation, such as CRP and serum amyloid A, in patients with atherosclerosis.
TNF-α inhibitors counteract insulin resistance and demonstrate an even higher protective effect against the development of diabetes or CV comorbidities in psoriasis patients. [47] Studies of PsA showed that anti-TNF-α treatment resulted in an anti-proatherogenic effect, and decreased LDL and triglyceride levels. For patients with associated comorbidities, the biologics approved for the treatment of psoriasis are valuable therapeutic options. [53] Pietrzak et al. in a review of CV aspects of psoriasis found an elevated risk of CV events in psoriatic patients in relation to non-psoriatic controls (odds ratio - OR 1.28). They suggested that the treatment of the inflammatory processes involved in the pathogenesis of both psoriasis and atherosclerosis may be beneficial in reducing the CV risk in psoriatic patients. [54]
Comorbidities: Recommendations for Screening
There is a need to establish recommendations for Indian patients with psoriasis for the early detection of comorbidities. As published in the National Psoriasis Foundation Clinical Consensus on Psoriasis Comorbidities, the American Heart Association recommends screening for CV risk factors as early as age 20. At the age of 40, the following risk factors need to be screened every 2 years: pulse, blood pressure (target < 120/80), body mass index < 25 kg/m2, waist circumference < 35 inches for women, <40 inches for men, total cholesterol < 200 mg/dL, HDL cholesterol = or > 50 mg/dL, LDL < 100 mg/dL and fasting blood glucose < 100 mg/dL. They also recommend smoking cessation, moderating alcohol intake, and exercising 3 times a week for 30 min. [55]
Conclusion
The awareness of comorbidities associated with psoriasis, particularly severe psoriasis, has led to a paradigm shift in the understanding of the disease and its management. Dermatologists should not just recognize and treat the signs and symptoms of psoriasis but should also screen patients to detect the existence of comorbid conditions such as PsA, metabolic syndrome, and CV disease. A multidisciplinary approach, with coordination between dermatologists and other specialists, is needed due to the systemic nature of inflammation in psoriatics. This will minimize co-medication, prevent overlap and improve compliance, improving the standards of care of psoriasis patients.
| 1. |
Onumah N, Kircik LH. Psoriasis and its comorbidities. J Drugs Dermatol 2012;11:s5-10.
[Google Scholar]
|
| 2. |
Fernandez-Torres RM, Paradela S, Fonseca E. Psoriasis in patients older than 65 years. A comparative study with younger adult psoriatic patients. J Nutr Health Aging 2012;16:586-91.
[Google Scholar]
|
| 3. |
Love TJ, Qureshi AA, Karlson EW, Gelfand JM, Choi HK. Prevalence of the metabolic syndrome in psoriasis: Results from the National Health and Nutrition Examination Survey, 2003-2006. Arch Dermatol 2011;147:419-24.
[Google Scholar]
|
| 4. |
Azfar RS, Gelfand JM. Psoriasis and metabolic disease: Epidemiology and pathophysiology. Curr Opin Rheumatol 2008;20:416-22.
[Google Scholar]
|
| 5. |
Gisondi P, Del Giglio M, Cozzi A, Girolomoni G. Psoriasis, the liver, and the gastrointestinal tract. Dermatol Ther 2010;23:155-9.
[Google Scholar]
|
| 6. |
Esposito M, Saraceno R, Giunta A, Maccarone M, Chimenti S. An Italian study on psoriasis and depression. Dermatology 2006;212:123-7.
[Google Scholar]
|
| 7. |
Russo PA, Ilchef R, Cooper AJ. Psychiatric morbidity in psoriasis: A review. Australas J Dermatol 2004;45:155-9.
[Google Scholar]
|
| 8. |
Kimball AB, Jacobson C, Weiss S, Vreeland MG, Wu Y. The psychosocial burden of psoriasis. Am J Clin Dermatol 2005;6:383-92.
[Google Scholar]
|
| 9. |
Rakhesh SV, D'Souza M, Sahai A. Quality of life in psoriasis: A study from south India. Indian J Dermatol Venereol Leprol 2008;74:600-6.
[Google Scholar]
|
| 10. |
Gaikwad R, Deshpande S, Raje S, Dhamdhere DV, Ghate MR. Evaluation of functional impairment in psoriasis. Indian J Dermatol Venereol Leprol 2006;72:37-40.
[Google Scholar]
|
| 11. |
Hayes J, Koo J. Psoriasis: Depression, anxiety, smoking, and drinking habits. Dermatol Ther 2010;23:174-80.
[Google Scholar]
|
| 12. |
Kimball AB, Wu EQ, Guérin A, Yu AP, Tsaneva M, Gupta SR, et al. Risks of developing psychiatric disorders in pediatric patients with psoriasis. J Am Acad Dermatol 2012;67:651-7.
[Google Scholar]
|
| 13. |
Golpour M, Hosseini SH, Khademloo M, Ghasemi M, Ebadi A, Koohkan F, et al. Depression and anxiety disorders among patients with psoriasis: A Hospital-Based Case-Control study. Dermatol Res Pract 2012;2012:381905.
[Google Scholar]
|
| 14. |
Devrimci-Ozguven H, Kundakci TN, Kumbasar H, Boyvat A. The depression, anxiety, life satisfaction and affective expression levels in psoriasis patients. J Eur Acad Dermatol Venereol 2000;14:267-71.
[Google Scholar]
|
| 15. |
Scharloo M, Kaptein AA, Weinman J, Bergman W, Vermeer BJ, Rooijmans HG. Patients' illness perceptions and coping as predictors of functional status in psoriasis: A 1-year follow-up. Br J Dermatol 2000;142:899-907.
[Google Scholar]
|
| 16. |
Gupta MA, Gupta AK. Depression and suicidal ideation in dermatology patients with acne, alopecia areata, atopic dermatitis and psoriasis. Br J Dermatol 1998;139:846-50.
[Google Scholar]
|
| 17. |
Kremers HM, McEvoy MT, Dann FJ, Gabriel SE. Heart disease in psoriasis. J Am Acad Dermatol 2007;57:347-54.
[Google Scholar]
|
| 18. |
Cassano N, Vestita M, Apruzzi D, Vena GA. Alcohol, psoriasis, liver disease, and anti-psoriasis drugs. Int J Dermatol 2011;50:1323-31.
[Google Scholar]
|
| 19. |
Mattoo SK, Handa S, Kaur I, Gupta N, Malhotra R. Psychiatric morbidity in vitiligo and psoriasis: A comparative study from India. J Dermatol 2001;28:424-32.
[Google Scholar]
|
| 20. |
Dogra S, Yadav S. Psoriasis in India: Prevalence and pattern. Indian J Dermatol Venereol Leprol 2010;76:595-601.
[Google Scholar]
|
| 21. |
Neimann AL, Shin DB, Wang X, Margolis DJ, Troxel AB, Gelfand JM. Prevalence of cardiovascular risk factors in patients with psoriasis. J Am Acad Dermatol 2006;55:829-35.
[Google Scholar]
|
| 22. |
Yang YW, Keller JJ, Lin HC. Medical comorbidity associated with psoriasis in adults: A population-based study. Br J Dermatol 2011;165:1037-43.
[Google Scholar]
|
| 23. |
Alberti KG, Zimmet P, Shaw J, IDF Epidemiology Task Force Consensus Group. The metabolic syndrome - A new worldwide definition. Lancet 2005;366:1059-62.
[Google Scholar]
|
| 24. |
Liu J, Grundy SM, Wang W, Smith SC Jr, Vega GL, Wu Z, et al. Ethnic-specific criteria for the metabolic syndrome: Evidence from China. Diabetes Care 2006;29:1414-6.
[Google Scholar]
|
| 25. |
Sterry W, Strober BE, Menter A, International Psoriasis Council. Obesity in psoriasis: The metabolic, clinical and therapeutic implications. Report of an interdisciplinary conference and review. Br J Dermatol 2007;157:649-55.
[Google Scholar]
|
| 26. |
Pietrzak A, Chodorowska G, Szepietowski J, Zalewska-Janowska A, Krasowska D, Hercogová J. Psoriasis and serum lipid abnormalities. Dermatol Ther 2010;23:160-73.
[Google Scholar]
|
| 27. |
Takahashi H, Iizuka H. Psoriasis and metabolic syndrome. J Dermatol 2012;39:212-8.
[Google Scholar]
|
| 28. |
Alsufyani MA, Golant AK, Lebwohl M. Psoriasis and the metabolic syndrome. Dermatol Ther 2010;23:137-43.
[Google Scholar]
|
| 29. |
Duarte GV, Follador I, Cavalheiro CM, Silva TS, Oliveira Mde F. Psoriasis and obesity: Literature review and recommendations for management. An Bras Dermatol 2010;85:355-60.
[Google Scholar]
|
| 30. |
Naldi L, Mercuri SR. Epidemiology of comorbidities in psoriasis. Dermatol Ther 2010;23:114-8.
[Google Scholar]
|
| 31. |
Duarte GV, Oliveira Mde F, Cardoso TM, Follador I, Silva TS, Cavalheiro CM, et al. Association between obesity measured by different parameters and severity of psoriasis. Int J Dermatol 2013;52:177-81.
[Google Scholar]
|
| 32. |
Boehncke WH, Boehncke S, Tobin AM, Kirby B. The 'psoriatic march': A concept of how severe psoriasis may drive cardiovascular comorbidity. Exp Dermatol 2011;20:303-7.
[Google Scholar]
|
| 33. |
Vena GA, Vestita M, Cassano N. Psoriasis and cardiovascular disease. Dermatol Ther 2010;23:144-51.
[Google Scholar]
|
| 34. |
Wakkee M, Thio HB, Prens EP, Sijbrands EJ, Neumann HA. Unfavorable cardiovascular risk profiles in untreated and treated psoriasis patients. Atherosclerosis 2007;190:1-9.
[Google Scholar]
|
| 35. |
Wu JJ, Poon KY, Channual JC, Shen AY. Association between tumor necrosis factor inhibitor therapy and myocardial infarction risk in patients with psoriasis. Arch Dermatol 2012;148:1244-50.
[Google Scholar]
|
| 36. |
Madanagobalane S, Anandan S. The increased prevalence of non-alcoholic fatty liver disease in psoriatic patients: A study from South India. Australas J Dermatol 2012;53:190-7.
[Google Scholar]
|
| 37. |
Binus AM, Han J, Qamar AA, Mody EA, Holt EW, Qureshi AA. Associated comorbidities in psoriasis and inflammatory bowel disease. J Eur Acad Dermatol Venereol 2012;26:644-50.
[Google Scholar]
|
| 38. |
Wolf N, Quaranta M, Prescott NJ, Allen M, Smith R, Burden AD, et al. Psoriasis is associated with pleiotropic susceptibility loci identified in type II diabetes and Crohn disease. J Med Genet 2008;45:114-6.
[Google Scholar]
|
| 39. |
Ludvigsson JF, Lindelöf B, Zingone F, Ciacci C. Psoriasis in a nationwide cohort study of patients with celiac disease. J Invest Dermatol 2011;131:2010-6.
[Google Scholar]
|
| 40. |
Gelfand JM, Shin DB, Neimann AL, Wang X, Margolis DJ, Troxel AB. The risk of lymphoma in patients with psoriasis. J Invest Dermatol 2006;126:2194-201.
[Google Scholar]
|
| 41. |
Paul CF, Ho VC, McGeown C, Christophers E, Schmidtmann B, Guillaume JC, et al. Risk of malignancies in psoriasis patients treated with cyclosporine: A 5 y cohort study. J Invest Dermatol 2003;120:211-6.
[Google Scholar]
|
| 42. |
Frentz G, Olsen JH. Malignant tumours and psoriasis: A follow-up study. Br J Dermatol 1999;140:237-42.
[Google Scholar]
|
| 43. |
Chiang YY, Lin HW. Association between psoriasis and chronic obstructive pulmonary disease: A population-based study in Taiwan. J Eur Acad Dermatol Venereol 2012;26:59-65.
[Google Scholar]
|
| 44. |
Dreiher J, Weitzman D, Shapiro J, Davidovici B, Cohen AD. Psoriasis and chronic obstructive pulmonary disease: A case-control study. Br J Dermatol 2008;159:956-60.
[Google Scholar]
|
| 45. |
Bañares A, Hernández-García C, Fernández-Gutiérrez B, Jover JA. Eye involvement in the spondyloarthropathies. Rheum Dis Clin North Am 1998;24:771-84.
[Google Scholar]
|
| 46. |
Lima FB, Abalem MF, Ruiz DG, Gomes Bde A, Azevedo MN, Moraes HV Jr, et al. Prevalence of eye disease in Brazilian patients with psoriatic arthritis. Clinics (Sao Paulo) 2012;67:249-53.
[Google Scholar]
|
| 47. |
Reich K. The concept of psoriasis as a systemic inflammation: Implications for disease management. J Eur Acad Dermatol Venereol 2012;26:3-11.
[Google Scholar]
|
| 48. |
Wakkee M, Meijer W, Neumann HA, Herings RM, Nijsten T. Psoriasis may not be an independent predictor for the use of cardiovascular and anti-diabetic drugs: A 5-year prevalence study. Acta Derm Venereol 2009;89:476-83.
[Google Scholar]
|
| 49. |
Cohen AD, Bonneh DY, Reuveni H, Vardy DA, Naggan L, Halevy S. Drug exposure and psoriasis vulgaris: Case-control and case-crossover studies. Acta Derm Venereol 2005;85:299-303.
[Google Scholar]
|
| 50. |
Gerdes S, Zahl VA, Knopf H, Weichenthal M, Mrowietz U. Comedication related to comorbidities: A study in 1203 hospitalized patients with severe psoriasis. Br J Dermatol 2008;159:1116-23.
[Google Scholar]
|
| 51. |
Alexandroff AB, Pauriah M, Camp RD, Lang CC, Struthers AD, Armstrong DJ. More than skin deep: Atherosclerosis as a systemic manifestation of psoriasis. Br J Dermatol 2009;161:1-7.
[Google Scholar]
|
| 52. |
Späh F. Inflammation in atherosclerosis and psoriasis: Common pathogenic mechanisms and the potential for an integrated treatment approach. Br J Dermatol 2008;159:10-7.
[Google Scholar]
|
| 53. |
Boehncke WH, Boehncke S, Schön MP. Managing comorbid disease in patients with psoriasis. BMJ 2010;340:b5666.
[Google Scholar]
|
| 54. |
Pietrzak A, Bartosi´nska J, Chodorowska G, Szepietowski JC, Paluszkiewicz P, Schwartz RA. Cardiovascular aspects of psoriasis: An updated review. Int J Dermatol 2013;52:153-62.
[Google Scholar]
|
| 55. |
Kimball AB, Gladman D, Gelfand JM, Gordon K, Horn EJ, Korman NJ, et al. National Psoriasis Foundation clinical consensus on psoriasis comorbidities and recommendations for screening. J Am Acad Dermatol 2008;58:1031-42.
[Google Scholar]
|
Fulltext Views
17,889
PDF downloads
4,753





