Translate this page into:
Comparative evaluation of NBUVB phototherapy and PUVA photochemotherapy in chronic plaque psoriasis
2 Department of Dermatology, Eras Lucknow Medical College, Lucknow, India
Correspondence Address:
Surabhi Dayal
18, Vikas Nagar, Rohtak, Haryana
India
| How to cite this article: Dayal S, M, Jain V K. Comparative evaluation of NBUVB phototherapy and PUVA photochemotherapy in chronic plaque psoriasis. Indian J Dermatol Venereol Leprol 2010;76:533-537 |
Abstract
Background: Psoralen UV-A (PUVA) is an established therapy for psoriasis, but there is a well-documenated risk of melanoma and nonmelanoma skin cancer. Narrow-band Ultraviolet-B (NBUVB) therapy has a lower carcinogenic risk, has equal therapeutic potential and is considerably safe in the long term than PUVA. Aim: The aim of present study was to compare the efficacy and side-effects of PUVA and NBUVB in chronic plaque psoriasis. Methods: Sixty patients of chronic plaque psoriasis were taken up for the study and were randomly divided into two groups of 30 each. They were well matched in terms of age, sex, psoriasis extent and pretreatment psoriasis area severity index (PASI) scoring. One group was treated with twice-weekly narrow-band UV-B (TL-01) phototherapy and the other group received twice-weekly oral 8-Methoxsalen PUVA for a period of 3 months. Results: Both the groups achieved >75% reduction in the PASI score or complete clearance at the end of 3 months, but PUVA group patients required significantly fewer number of treatment sessions and fewer number of days to clear the psoriasis as compared to the NBUVB group, while the mean cumulative clearance dose and adverse effects were significantly lower in the NBUVB group. Conclusion: We concluded that PUVA group patients achieved a faster clearance, but the adverse effects were significantly lower in the NBUVB group.Introduction
Psoralen UV-A (PUVA) is an established therapy for psoriasis. [1] There is, however, increasing concern about the long-term safety of PUVA because of the now well-documented increased risk of nonmelanoma skin cancer and, more recently, reported risk of melanoma. [2],[3],[4],[5] Studies of wavelength-dependence of the response of psoriasis to UVB (290-320 nm) phototherapy have led to the development of the TL-01 fluorescent UVB lamp in which 83% of the UV emission is at 311 ± 2 nm, a spectral region known to be effective at clearing psoriasis. [6] The development of the TL-O1 lamp with higher therapeutic potential than broad-band UVB source and less carcinogenic risk than PUVA has led to the continuously growing use of NBUVB therapy in psoriasis. [7],[8]
Although PUVA therapy of psoriasis is clearly more effective than conventional broad-band phototherapy, [9],[10],[11],[12] there are few studies available comparing PUVA with the new modality of NBUVB phototherapy. [13],[14],[15],[16] These studies have suggested that NBUVB may have therapeutic efficacy equal to PUVA. If this is confirmed, this would be of considerable importance because NBUVB is likely to be devoid of side-effects and will be considerably safe in the long term than PUVA. Therefore, we planned to undertake a trial comparing twice-weekly PUVA and NBUVB phototherapy for the treatment of psoriasis in terms of efficacy, time to clear and adverse effects.
Methods
Sixty patients of chronic plaque psoriasis, with at least 25% of the body surface area involvement (according to rule of nine [17] ), attending the outpatient department of PGIMS, Rohtak, were taken up for the study.The study was approved by the ethical committee. The patients were selected between Feb 2004 and May 2005. The duration of therapy was 3 months or till the patients achieved 75% reduction in the psoriasis area severity index (PASI) score, whichever was earlier. Exclusion criteria included age younger than 16 years or more than 60 years, pregnancy or lactation, patients who had taken any specific antipsoriatic treatment within the last 4 weeks, renal or hepatic disease, history suggestive of photosensitivity, previous failure or intolerance to phototherapy, any history suggestive of malignant melanoma or squamous cell carcinoma or polymorphic light eruptions or on immunosuppressive agents.
PASI [18] and detailed dermatological examination was performed in all the patients. The patients were then randomly divided into two groups of 30 each. To ensure proper randomization, patients who met the inclusion criteria and were reporting on Monday, Wednesday and Friday were administered NBUVB therapy and those who reported on Tuesday, Thursday and Saturday were administered UVA therapy. Group-I included 30 patients who were given NBUVB phototherapy and Group-II included 30 patients who were given 8-methoxsalen PUVA therapy. Informed written consent was obtained prior to both NBUVB and PUVA therapy.
Patients of Group-1 were treated with NBUVB as a monotherapy in a phototherapy unit (V-care NBUVB therapy unit) as source of irradiation. A standard initial NBUVB dose of 280 mj/cm 2 for skin type IV or V (representative of our local population) was started. [19],[20]
Patients of Group-II were given Oral-8MOP crystalline tablets at a dose of 0.6 mg/kg body weight 2 h before UV-A exposure and were then exposed to UV-A irradiation in a UV-A phototherapy unit (V care UVA therapy unit). Standard initial UV-A dose for the Indian skin type IV or V of 2 J/cm 2 was started. [21],[22]
In both the groups, phototherapy was administered twice weekly on Monday and Thursday (i.e., on nonconsecutive days). The irradiation dose was increased by 20% of the previous dose on each subsequent visit. The grade of erythema, pigmentation, pruritus and any adverse effect was recorded at each visit. If symptomatic erythema (burning, pain or blistering) developed, the irradiation dose was decreased by 50% of the burning dose and, thereafter, the dose was increased by 10% on the subsequent visit. [16] During phototherapy, the patients wore UV-protective goggles and the genitalia was shielded.
All patients of both the groups were examined by the same dermatologist at each visit up to 3 months. PASI scoring was carried out again at 4 weeks, 8 weeks and 12 weeks of therapy. The patient was considered in remission when there was 75% reduction in PASI score. [23] Results were statistically analyzed using the Mann-Whitney test. [16]
All the patients were monitored for adverse effects. The grade of erythema, tanning, pruritus and any other adverse effect was recorded at each visit.
Results
Of the 60 patients studied, 30 received NBUVB (TL-O1) phototherapy (Group-1) and 30 patients received PUVA therapy (Group-II). The two groups were well matched for age, sex, skin type, involvement of body surface area and PASI score. The maximum number of patients, in both narrow-band UV-B and PUVA group, were seen in the age group of 21-40 years. The minimum age taken was 16 years and the maximum was 55 years. The mean age of NBUVB was 32.1 years and the mean age of the PUVA group was 32.45 years. The difference in ages of two groups was not statistically significant (P > 0.05). Therefore, the two groups were comparable in terms of age of the patients. In the NBUVB group, there were 18 males and 12 females, with a male:female ratio of 3:2, and in the PUVA group, there were 22 males and eight females, with a male:female ratio of 2.7:1. In both the groups, males outnumbered females.
In the NBUVB group, 21 patients had involvement of body surface area between 25% and 50%, and nine patients had involvement of body surface area between 50% and 75%. In the PUVA group, 19 patients had involvement of body surface area between 25% and 50% and 11 patients had involvement of body surface area between 50% and 75%. In both the groups, all the patients had involvement of at least 25% of the body surface area. In the NBUVB group, the total duration of disease at the time of inclusion in this study ranged between 6 months and 27 years. In the PUVA group, the total duration of disease at the time of inclusion ranged between 6 months and 30 years.
PASI score in psoriatic patients included in the NBUVB group before start of therapy ranged from 12.2 to 30.6 (mean, 16.82 ± 3.90 SD). PASI in the psoriatic patients included in the PUVA group before start of therapy ranged from 16.4 to 34.8 (mean, 21.6 ± 4.42 SD). The difference in ages of the two groups was not statistically significant (P > 0.05). Therefore, the psoriatic patients included for the study in the two groups were comparable in terms of PASI scoring.
At the end of 3 months of therapy, all the patients of both the groups achieved >75% clearance or complete clearance. The posttreatment PASI score of patients who were given NBUVB therapy ranged from 0 to 3.2 (mean, l.6 ± 1.2), while the posttreatment PASI scoring of patients who were given PUVA ranged from 0 to 2.6 (mean, 1.39 ± 0.78). The difference in PASI scoring after 3 months of therapy between the NBUVB group and the PUVA group was not statistically significant (P > 0.05) [Table - 1].
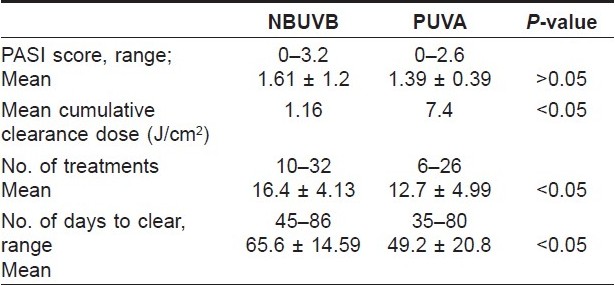
The mean cumulative dose required for clearance in the NBUVB group was 1.16 J/cm 2 while in the PUVA group it was 7.4 J/cm 2 . The cumulative clearance dose was statistically significantly lower in the NBUVB group than in the PUVA group (P < 0.05) [Figure - 1] and [Figure - 2].
 |
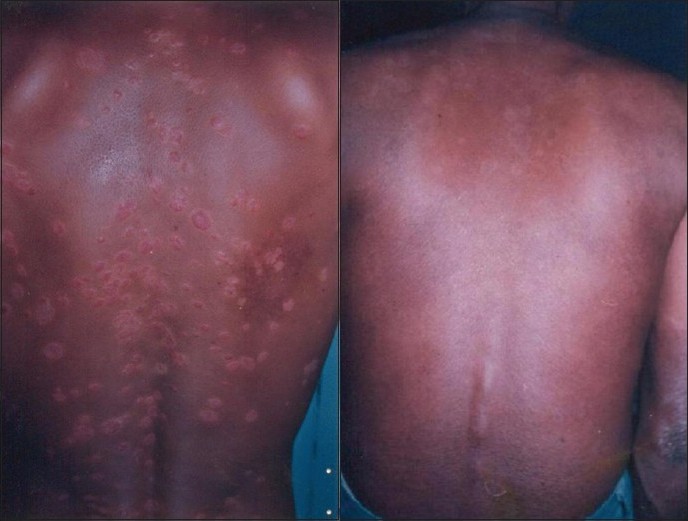
| Figure 1 :(a) Before Psoralen UV-A therapy Figure 1b: After 78 doses of Psoralen UV-A therapy |
 |
| Figure 2 :(a) Before NBUVB therapy Figure 2b: After 87 doses of NBUVB therapy |
In patients receiving NBUVB phototherapy, the number of treatments required for clearance of psoriasis ranged from 10 to 32 (mean, 16.4 ± 4.13), which were statistically significant higher (P < 0.05) than the number of treatments required for clearance in patients receiving PUVA, which ranged from 6 to 26 (mean, 12.7 ± 4.9). The number of days to clear psoriasis in patients receiving NBUVB therapy (range, 45-86 days; mean, 65.6 + 14.09) was also higher than that in the PUVA group (range, 35-80 days; mean, 49.2 + 20.8). The difference was statistically significant (P < 0.05).
As far as adverse effects were concerned, all patients (100%) in both the groups developed grade-1 erythema. However, grade-II erythema was observed more frequently in the PUVA group (70%) as compared to the NBUVB group (40%). Eighty percent of the PUVA group patients had pruritus and 75% experienced nausea and vertigo, while in the NBUVB group, only 30% experienced nausea and vertigo. Ninety percent of the PUVA group patients complained of headache while in the NBUVB group, only 45% complained of headache. Patients in the PUVA group experienced more diffuse hair fall (70%) as compared to those in the NBUVB group (30%).
Discussion
The newer modality, NBUVB phototherapy, is a potential advance in UVB-based phototherapy and is increasingly being used in the treatment of psoriasis. With regards to the relative therapeutic effectiveness of NBUVB and PUVA, however, only sparse data are available. [13],[14],[15],[16] There are few studies comparing PUVA with this new modality of NBUVB, and most of these earlier studies have compared these two modalities using half-body comparison.
The present study was therefore undertaken in which we compared whole-body NBUVB phototherapy with whole-body PUVA in chronic plaque psoriasis. The patients were randomly divided into two groups and were treated with either twice-weekly NBUVB phototherapy or twice-weekly 8-MOP PUVA for 3 months. Both groups were well matched in terms of age, sex, duration of illness and pretreatment PASI scoring.
At the end of 3 months of therapy, all the patients in both groups were totally clear of psoriasis and the difference in the PASI scoring between the two groups was not statistically significant (P > 0.05). All the patients in both the groups achieved >75% reduction in PASI score. However, the mean cumulative clearance dose in the NBUVB group was statistically significantly lower than that for the PUVA group (P < 0.05). In our study, we found that the number of treatments and number of doses required for clearance in the PUVA group were statistically fewer than that required in the NBUVB group (P < 0.05).
Our findings are in agreement with the study performed by Gordan et al.,[14] who randomized 100 patients of plaque psoriasis to receive either PUVA or NBUVB, and have shown that clearance of psoriasis was faster with fewer number of treatments and days to clear in significantly greater proportion of patients using PUVA as compared to NBUVB. They have also observed that the cumulative clearance dose for the NBUVB group was significantly lower than the PUVA group.
In an open, randomized and controlled study conducted by Markham et al.,[16] 54 chronic plaque psoriatics were treated with an equierythemogenic dosage of either thrice-weekly TL-O1 NBUVB or twice-weekly 8-MOP PUVA until completely clear. They concluded that patients in the PUVA group required significantly fewer treatments than patients in the NBUVB group, which is similar to our finding. However, they also showed that there was no statistically significant difference in the number of days to clear the psoriasis between the two groups. This is contrary to observation of our study, in which we found that the number of days to clear psoriasis was significantly higher in the NBUVB group. This may be due to the fact that, in our study, patients of both the groups received twice-weekly therapy. We chose to give twice-weekly therapy in both the groups to make a direct comparison of the two treatment modalities.
Advese effects like grade-I erythema developed in all patients in both groups, indicating that both treatment modalities are erythemogenic. But, grade-II erythema and other adverse effects like nausea, vertigo, headache, pruritus and diffuse hair fall was observed more commonly in the PUVA group. This is in agreement with Markham and Collins, [24] who have also reported fewer side-effects with NBUVB (TL-01) as compared to PUVA in an audit of adverse effects of oral 8-MOP PUVA and NBUVB (TL-0l) in the management of chronic plaque psoriasis.
In conclusion, our study has shown that patients of both NBUVB and PUVA groups achieved >75% clearance or complete clearance at the end of 3 months of therapy, but patients in the PUVA group achieved faster clearance, required significantly fewer number of treatment sessions and fewer number of days to clear psoriasis as compared to the NBUVB group. However, the mean cumulative clearance dose and adverse effects were lower in the NBUVB group than in the PUVA group.
| 1. |
Parrish JA, Stern RS, Pathak MA, Fitzpatrick TB. Photochemotherapy of skin disease. In: Regan JD, Parrish JA, editors. The science of photomedicine. New York: Plenum Press; 1982. p. 595-623.
[Google Scholar]
|
| 2. |
Lindelφf B, Sigurgeirsson B, Tegner E, Larkφ O, Johannesson A, Berne B, et al. PUVA and cancer: Large scale epidemiological study. Lancet 1991;338:91-3.
[Google Scholar]
|
| 3. |
Stern RS, Laird N. The carcinogenic risk of treatments for severe psoriasis. Cancer 1994;73:2759-64.
[Google Scholar]
|
| 4. |
Lever LR, Farr PM. Skin cancers or premalignant lesions occur in half of high dose PUVA patients. Br J Dermatol 1994;131:215-9.
[Google Scholar]
|
| 5. |
Stern RS; PUVA Follow up study. The risk of melanoma in association with long term exposure to PUVA. J Am Acad Dermatol 2001;44:755-61.
[Google Scholar]
|
| 6. |
arrish JA, Jaenicke KF. Action spectrum of phototherapy of psoriasis. J Invest Dermatol 1981;76:359-62.
[Google Scholar]
|
| 7. |
Conven TR, Burack LH, Gilleaudeau R, Keogh M, Ozawa M, Krueger JG. Narrow band UV-B produces superior clinical and histopathological resolutions of moderate -to - severe psoriasis in patients compared with broad band UV-B. Arch Dermatol 1997;133:1514-22.
[Google Scholar]
|
| 8. |
De Gruijl FR. Long term side effects and carcinogenesis risk in UVB therapy. In Hongismann H, Jori G, Young AR, editors. The Fundamental Basis of Phototherapy, OEMF spa, Italy 1996. p. 153-70.
[Google Scholar]
|
| 9. |
Boer J, Hermans J, Schothorst AA, Suurmond D. Comparison of phototherapy (UV-B) and photochemotherapy (PUVA) for clearing and maintainance therapy for psoriasis. Arch Dermatol 1984;120:52-7.
[Google Scholar]
|
| 10. |
Brenner W, Jaschke E, Honigsmann H. UV-B phototherpie in psoriasis. Z Hautkr 1983;58:1113-24.
[Google Scholar]
|
| 11. |
Hongisman H, Fritsch P, Jaschke E. UV- therapie der psoriasis. Halbseitenvergleic zwischen oraler photochcmotttcrapie (PUVA) and selektivcr UV-phototherapie (SUP), Z Hauktr 1977;52:1078-82.
[Google Scholar]
|
| 12. |
Morison WL. Combination of methoxsalen and ultravioletB (UVB) versus UVB radiation alone in treatment of psoriasis: a bilateral comparison study. Photodermatol Phtoimmunol Photomed 1995;11:6-8.
[Google Scholar]
|
| 13. |
Van Welden H, Baart de la Faille H, Young E, van der Leun JC. Comparison of narrowband UV-B phototherapy and PUVA photochemotherapy in the treatment of psoriasis. Acta Derm Venereol 1990;70:212-5.
[Google Scholar]
|
| 14. |
Gordon PM, Diffey BL, Matthews JN, Farr PM. A randomized comparison of narrow-band TL-O1 phototherapy and PUVA photochemotherapy for psoriasis. J Arn Acad Dermatol 1999;41:728-32.
[Google Scholar]
|
| 15. |
Tanew A, Radakovic-Fijan S, Schemper M, Hφnigsmann H. Narrowband UV-B phototherapy vs photochemotherapy in the treatment of chronic plaque type psoriasis. Arch Dermatol 1999;135:519-24.
[Google Scholar]
|
| 16. |
Markham T, Rogers S, Collins P. Narrowband UV-B (TL-0 l) Phototherapy vs Oral 8-Methoxypsoralen Psoralen-UV-A for the treatment of chronic plaque psoriasis. Arch Dermatol 2003;139:325-8.
[Google Scholar]
|
| 17. |
Knaysi GA, Crikelair GF, Cosman B. The rule of nines: its history and accuracy. Plast Reconstr Surg 1968;41:560-3.
[Google Scholar]
|
| 18. |
Fredicksson T, Pettersson U. Severe psoriasis, oral therapy with a new retinoid, Dermatologica 1978;157:238-44.
[Google Scholar]
|
| 19. |
Dogra S, Kanwar AJ. Narrowband UV-B phototherapy in dermatology. Indian J Dermatol Venereol Leprol 2004;70:205-9.
[Google Scholar]
|
| 20. |
British Photodermatology Group: An appraisal of narrow band UVB phototherapy: British Photodermatology Group Workshop Report (April, 1996). Br J Dermatol 1997;137:327-30.
[Google Scholar]
|
| 21. |
Melski JW, Tanenbaum L, Parrish JA, Fitzpatrick TB, Bleich HL. Oral Methoxsalen photochemotherapy for the treatment of psoriasis: A cooperative clinical trial. J Invest Dermatol 1977;68:328.
[Google Scholar]
|
| 22. |
Henseler T, Wolff K, Hφnigsmann H, Christopher E. The European PUVA study (EPS): Oral 8-methoxypsoralen photochemotherapy of psoriasis: Cooperative study among 18 European centers. Lancet 1981;1:853-7.
[Google Scholar]
|
| 23. |
Carlin CS, Feldman SR, Krueger JG, Menter A, Krueger GG. A 50% reduction in the Psoriasis Area and Severity Index PASI 50 is a clinically significant end point in the assessment of psoriasis. J Am Acad Dermatol 2004;50:859-66.
[Google Scholar]
|
| 24. |
Markham T, Collins P. An audit of adverse effects of oral 8-methoxypsoralen ultraviolet-A and narrowband UVB phototherapy in the management of chronic plaque psoriasis. Br J Dermatol 2001;145:40-76.
[Google Scholar]
|
Fulltext Views
4,437
PDF downloads
1,940





