Translate this page into:
Comparative study of the efficacy of azathioprine, dapsone, and NB-UVB phototherapy as steroid-sparing modalities in generalised lichen planus
Corresponding author: Dr. Mithra S, Department of Dermatology, Venereology and Leprosy, Government Stanley Hospital, Chennai, India. drmithravasanthvignesh@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Mithra S, Parimalam K, Sowmiya R. Comparative study of the efficacy of azathioprine, dapsone, and NB-UVB phototherapy as steroid-sparing modalities in generalised lichen planus. Indian J Dermatol Venereol Leprol. 2025;91:59-64. doi: 10.25259/IJDVL_383_2023
Abstract
Background
Generalised lichen planus (GLP) is a chronic disease with an overall prevalence of 1% requiring longer treatment. Limited studies are available on GLP and its treatment in the literature, unlike oral lichen planus.
Objectives
To determine the best steroid-sparing treatment modality for GLP by comparing the efficacy, response, safety, side effects, and remission with azathioprine, dapsone, and narrowband UV-B (NB-UVB) along with their impact on itching severity and life quality.
Methodology
Open-label, prospective, comparative, interventional study on generalised lichen planus patients treated with systemic steroids along with one of three steroid-sparing modalities. Totally 90 patients were studied including 30 patients each who received azathioprine (Group A), dapsone (Group B), and narrow band UVB (NB-UVB) (Group C), respectively, for 16 weeks. Itch severity index (ISI) and Dermatology life quality Index (DLQI) were assessed at baseline and week 24. All patients received oral prednisolone until there was no more active disease. Response was assessed in terms of occurrence of new lesions, flattening of lesions, post-inflammatory hyperpigmentation (PIH), and grading of lesions two weeks once for 6 months followed by six months of follow-up after treatment completion.
Results
Females outnumbered males in all 3 groups. Mean patient ages (34, 38, and 34) and the presence of one or more co-morbidities (50%, 42.3%, 37.5%) in Groups A, B, and C, respectively, were comparable. ISI and DLQI improvement at 24 weeks were greatest with NB-UVB, followed by azathioprine and dapsone in that order; the differences in improvement between groups showed high statistical significance. At week 24, occurrence of new lesions (0%, 0%, 3.8%), flattening (100% – all groups), PIH (100% – all groups), grade 3 lesions i.e. poor response, resolution of 20-50% of lesions (7.1%, 11.5%, 0%), grade 2 lesions i.e. partial response, resolution of 50-90% of lesions (35.7%, 76.9%, 8.3%) and grade 1 lesions i.e. complete response, resolution of >90% lesions (57.1%, 11.5%, 91.3%) were noted in Groups A, B and C, respectively; the differences in the extent of resolution of lesions between the groups were highly significant statistically. Remission was seen in 100%, 76.9%, and 87.5% in Groups A, B, and C, respectively, after six months.
Limitations
The sample size was small. Only 3 treatment options were compared in this study but many more options have been used for lichen planus. Long term follow-up is required.
Conclusions
NB-UVB with oral steroids showed a better response in terms of improvement in DLQI, ISI, disease control, and side effects than azathioprine and dapsone. Azathioprine showed a faster response and more prolonged remission. Dapsone showed poor response with multiple side effects.
Keywords
azathioprine
dapsone
DLQI
generalised lichen planus
ISI
narrowband UV-B
Introduction
Lichen planus (LP) is an autoimmune skin disease that can affect the skin or mucosae. It is associated with various co-morbidities and is characterised by poor treatment response, tendency to relapse, and impaired quality of life due to intense itching and post-inflammatory hyperpigmentation (PIH).1–3
Generalised lichen planus (GLP) can affect the skin, oral mucosa, genital mucosa, scalp, nails, or all these sites, with an overall prevalence of 1% in the general population.4 When there is extensive cutaneous involvement, it shows a minimal tendency to heal spontaneously and warrants a longer duration of treatment for disease control. Unlike oral LP, the available literature on treatment options for extensive or generalised cutaneous LP is very limited.
The available case reports highlight the good response of cutaneous lichen planus (LP) to azathioprine, itraconazole, and mycophenolate mofetil.5–7 Corticosteroids have been widely used in the initial period of treating LP.8–10
In a report, seven of nine Indian lichen planus (LP) patients showed a complete response to azathioprine.11 The efficacy of dapsone has been reported to be 18% higher than corticosteroids.12 Studies from the literature have cited that narrow band ultraviolet-B (NB-UVB) was more effective than six weeks of low-dose prednisolone.13
There is a need to determine the best steroid-sparing treatment modality for generalised LP. The aim of this study was to compare the efficacy, safety, and duration of remission as well as the impact on itching severity and life quality when azathioprine, dapsone, and NB-UVB are used along with systemic steroid therapy for generalised LP.
Methodology
A randomised, prospective, comparative, open label interventional study was conducted over one year after institutional ethical committee approval.
Patients of any age and sex with generalised lichen planus with cutaneous lesions involving at least 20% body surface area (BSA) were included. Diagnosis was mainly clinical but in five doubtful cases, skin biopsy was performed.
The exclusion criteria were pregnancy, photosensitivity, active infections, or immunosuppressed states such as cancer or ongoing immunosuppressive therapy. Informed consent for treatment and publication was obtained from all patients included in the study. Guardian’s consent was obtained for patients below 18 years.
The study flow chart is depicted in Figure 1. There were 30 patients in each treatment group A, B and C; allocation to groups was done by block randomisation methods. Concealment of allocation was achieved using a sequentially numbered, opaque, sealed envelope (SNOSE).
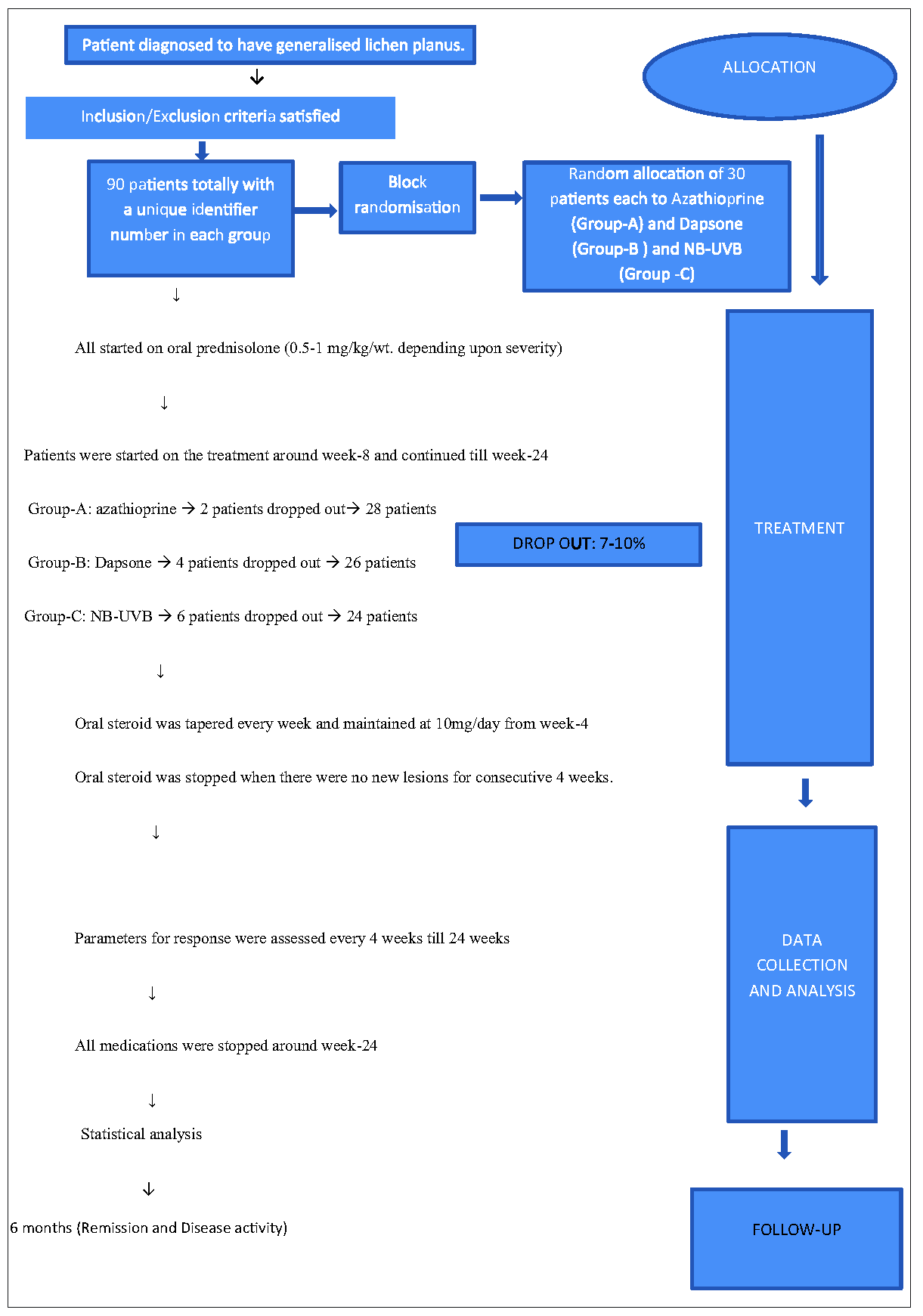
- Flow diagram of the study.
All patients initially received oral corticosteroids (0.5-1 mg/kg/day), which were tapered over 4 weeks after which they were maintained at 10 mg/day for several weeks. Azathioprine (2 mg/kg/day), dapsone (2 mg/kg/day), and NB-UVB (twice weekly on non-consecutive days) were started concurrently around week 8 and continued till week 24 for Groups A, B, and C, respectively. Oral steroids were stopped around week 12 to week 18 for all the patients when no new lesions had developed for four consecutive weeks. All treatments were stopped at 24 weeks and patients underwent a further 6 months follow up. During the six months of follow-up, the absence of new lesions and lack of recurrence of old lesions was considered remission.
The response was assessed every two weeks for 24 weeks, and routine investigations were done every four weeks.
Itch severity index (ISI) using a 12-item pruritus severity score (PSS) and dermatology life quality index (DLQI) were scored at baseline and week 24. Patients below 16 years were not included in the assessment of DLQI. All three groups were assessed based on the following parameters: reduction in itching using ISI, improvement in DLQI, appearance and number of new lesions, and changes in pre-existing lesions: post-inflammatory hyperpigmentation or hypopigmentation (PIH) and flattening. Grading of total lesions (pre- and post- treatment) was done. Grade 1: complete response (disappearance of >90% lesions), Grade 2: partial response (disappearance of at least 50% lesions), Grade 3: poor response (improvement in 20%–50% lesions) and Grade 4: no response (<20% lesion reduction).
The collected data were analysed with IBM SPSS statistics software 23.0 version. To describe the data, descriptive statistics - frequency analysis and percentage analysis were used for categorical variables; mean and standard deviation (SD) were used for continuous variables. One-way ANOVA was used to compare the continuous variables between groups. The data were normally distributed, and normality was confirmed using Shapiro-Wilk’s test. The chi-square test was used to compare the categorical data between groups. The probability value (p-value) of 0.05 and below was considered to be significant for all statistical analyses.
Results
After excluding the drop-outs, 28, 26, and 24 patients who received azathioprine, dapsone, and NB-UVB, respectively, were considered for statistical analysis as per protocol.
Baseline demographics with clinical characteristics of each group are shown in Table 1. The age ranges were 6–65, 13–70, and 12–70 years in patients who received azathioprine, dapsone, and NB-UVB, respectively. Female patients were more in all the groups (p-value 0.125). There was no statistically significant difference in the occurrence of new lesions among the groups at 24 weeks (p-value 0.363). p-values could not be calculated for flattening and PIH as the parameters were subjective and no grading scales were used.
| Participant characteristics | Result | |
|---|---|---|
| Age (years) Group A, B, C | 34, 38, 34 | |
| Sex: Group A, B, C | ||
| Male | 42.9%, 30.8%, 16.7% | |
| Female | 57.1%, 30.8%, 16.7% | |
| Study groups | Lesions-Grade 4 (Before treatment) | Lesions-Grade 4 (After treatment) |
| Group-A (Azathioprine) | 3 (12.5%) | 4 (16.7%) |
| Group B (Dapsone) | 15 (62.5%) | 14 (58.3%) |
| Group-C (NB-UVB) | 6 (25.0%) | 6 (25.0%) |
In this study, 50% (14), 42.3% (11), and 37.5% (9) patients had one or more co-morbidities, (p-value 0.65, not significant) among patients on azathioprine, dapsone, and NB-UVB, respectively. Co-morbidities like Type-2 diabetes mellitus (2, 5, 3), systemic hypertension (3, 2, 1), dyslipidaemia (2, 4, 4), hypothyroidism (2, 2, 0), psychiatry disorder (0, 1, 0), bronchial asthma (1, 0, 0), obesity (2, 2, 0), coronary heart disease (0, 1, 0) and seizure disorder (0, 1, 0) were present in the above number of patients in the azathioprine, dapsone, and NB-UVB groups, respectively.
Table 2 and Figure 2 show the details of improvement in ISI and DLQI scores, which was greatest with NB-UVB followed by azathioprine and then dapsone. The differences in improvement between the groups at week 24 were highly statistically significant (p-value 0.0005).
| Analysis of variance | ||||||
|---|---|---|---|---|---|---|
| Sum of Squares | df | Mean Square | F | Sig. | ||
| ISI0 | Between Groups | 5.318 | 2 | 2.659 | .529 | .591 |
| Within Groups | 376.798 | 75 | 5.024 | |||
| Total | 382.115 | 77 | ||||
| DLQI0 | Between Groups | 11.824 | 2 | 5.912 | 1.682 | .193 |
| Within Groups | 263.663 | 75 | 3.516 | |||
| Total | 275.487 | 77 | ||||
| ISI24 | Between Groups | 71.135 | 2 | 35.567 | 25.348 | .0005 |
| Within Groups | 105.237 | 75 | 1.403 | |||
| Total | 176.372 | 77 | ||||
| DLQI24 | Between Groups | 58.299 | 2 | 29.149 | 27.819 | .0005 |
| Within Groups | 78.586 | 75 | 1.048 | |||
| Total | 136.885 | 77 | ||||
ISI0 and DLQIO represents ISI and DLQI score at baseline. ISI24 and DLQI24 represents ISI and DLQI score at week 24.
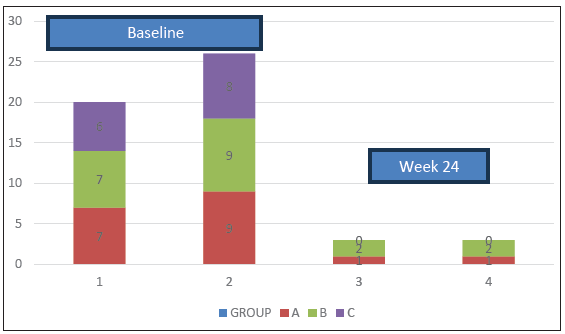
- Bar-1 depicts the baseline mean Itch Severity Index (ISI) scores and Bar-3 depicts mean ISI scores at week 24. Bar-2 and Bar-4 depict baseline and week-24 Dermatology Life Quality Index (DLQI) scores respectively.
At week 24, ISI scores in patients on azathioprine, dapsone, and NB-UVB, respectively, were as follows (the score value is given, followed by the number of patients with that score in brackets): lowest – 0 (27), 0 (25), 0 (22), highest – 4 (1), 8 (1), 1 (2). Similarly, DLQI scores at week 24 in the 3 groups respectively were: lowest – 0 (27), 0 (25), 0 (22), highest – 4 (1), 6 (1), 1 (2), At week 24, all 3 groups showed similar findings in terms of appearance of new lesions (0%, 3.8%, 0%), flattening of old lesions and PIH (all three groups – 100% each). At week 24, the lesion grading for all 3 groups were as follows - Grade 1 (complete response, resolution of >90% lesions): Group A - 57.1% (16), Group B - 11.5% (3), Group C - 91.3% (22); Grade 2 (partial response, resolution of 50-90% of lesions) Group A - 35.7% (10), Group B - 76.9% (20), Group C - 8.7% (2); Grade 3 (poor response, resolution of 20-50% of lesions): Group A - 7.1% (3), Group B - 11.5% (3), Group C - 0% and Grade 4 (no response, <20% lesion reduction): none in all 3 groups. Table 3 shows the grading of lesions in each group at weeks 0 and 24, and there was statistical significance between the groups at week-24 (p-value 0.0005).
| Group | Week | Grade-1 | Grade-2 | Grade-3 | Grade-4 |
|---|---|---|---|---|---|
| A (Azathioprine) | 0 | 0 | 0 | 1 | 27 |
| 24 | 16 | 10 | 2 | 0 | |
| B (Dapsone) | 0 | 0 | 0 | 0 | 26 |
| 24 | 3 | 20 | 3 | 0 | |
| C (NB-UVB) | 0 | 0 | 0 | 0 | 24 |
| 24 | 22 | 2 | 0 | 0 |
(Week 0) and Week- 24. Grade 1: complete response (disappearance of >90% lesions), Grade 2: partial response (disappearance of at least 50% lesions), Grade 3: poor response (improvement in 20%–50% lesions), and Grade 4: no response (<20% lesion reduction)
The side effects noted were weight gain (2, 2, 0), altered taste sensation (2, 1, 0), herpes labialis (1, 0, 0), herpes zoster (0, 0, 1), vomiting (1, 0, 0), hyperbilirubinemia (0, 3, 0), neutropenia (2, 0, 0), acneiform eruptions (4, 2, 0) and ophthalmic complaints (0, 0, 1) in patients who received azathioprine, dapsone, and NB-UVB, respectively. On follow up at 6 months (after week-24), remission was noted in 100% (26), 76.9% (21), and 87.5% (20) of patients who received azathioprine, dapsone, and NB-UVB, respectively; longer remission was observed in the azathioprine group compared to dapsone and NB-UVB.
Discussion
In this study, there was female predominance (F=54, M=24), with the predominent affection of middle age group (30–45 years), similar to other studies. Different age groups were distributed equally in all groups with a p-value of 0.125.14,15
Two pairs of familial cases were diagnosed.16,17 One pair was excluded because they were lost to follow-up, and the other pair, mother and daughter, responded well to NB-UVB and azathioprine, respectively.
Itch severity was assessed by a 12-item pruritus severity score (PSS).18 ISI and DLQI scores were better in Group C>A>B. Despite twice weekly visits to the hospital for NB-UVB, the absence of the need to take daily medications was an advantage.
Oral steroids helped in controlling new lesions in all groups. Few patients developed 1–3 new lesions when the steroid was stopped, highlighting that the steroid’s role was significant in controlling disease activity.19 The time taken to stop steroids varied between patients, and it was stopped approximately by week 12 for all patients, in contrast to Atzmony et al., who showed a complete response at six weeks.2
Azathioprine, dapsone, and NB-UVB were started around week 8 when patients were on oral prednisolone (10 mg/day). The oral steroid was stopped around week 12 in all three groups, and there was no significant difference in the duration of steroid requirement.
Group A stopped developing new lesions from week 12, similar to the observations of Verma et al., who found that azathioprine takes 4–6 weeks to act.20 NB-UVB consistently acted better in halting the appearance of new lesions from the first week of the treatment itself. Dapsone took two weeks to act,21 and its poorer response could be due to its better action on oral compared to cutaneous lesions.22 New lesions stopped appearing earliest in Group C followed by Groups A and B, respectively; these differences were not statistically significant.
The extent of flattening of lesions was equal in all groups, and the statistical difference could not be calculated. Post-inflammatory hyperpigmentation can be considered as a response and marker of disease inactivity.2 Assessment of flattening of lesions and PIH could vary depending on individual’s perceptions, with no standard grading method. Hence, they were considered a response parameter but not compared between groups. The observer noted that PIH was better in the following order: Group C>A>B.
In all groups, cutaneous lesions in the lower limbs responded slower, and hypertrophic lesions, predominantly in the lower limb, did not respond.23 These persistent hypertrophic lesions usually require intra-lesional corticosteroids due to failure of response to most systemic therapies, similar to this study.
Regarding the grading of lesions, the initial response was faster with azathioprine in reducing the lesion number by week-12. In contrast, NB-UVB was slower by four weeks but outperformed at the end, although both required approximately six weeks for response. This delay in NB-UVB could be due to reduced frequency to twice weekly in this study for better compliance compared to thrice weekly by Iraji et al.24
Two patients in Groups A and B complained of significant weight gain (>10 kg), and two out of four were adolescents. In Group A, two patients with altered taste sensation improved after stopping steroids, only one out of two patients with neutropenia recovered during follow-up, and one patient was excluded due to persistent vomiting with azathioprine despite treatment.
In Group B, hyperbilirubinemia was the major adverse effect seen in dapsone-treated patients with multiple co-morbidities. The most common co-morbidity present in these patients was dyslipidemia. Altered liver function tests (LFT) normalised after stopping dapsone. In Group C, all side effects were manageable.
Patients with multiple co-morbidities were slightly higher in the dapsone group, which may explain poor response or hyperbilirubinemia.
NB-UVB targets almost all the proposed pathogenetic mechanisms of lichen planus specifically, including apoptosis of keratinocytes and T-lymphocytes, altering antigen response and Langerhans cells depletion, decreasing NK cells, justifying its good response in cutaneous LP compared to azathioprine, which targets T-cell, B-cell activity and antigen number. Dapsone has shown poor response in disease control, adverse effects, and PIH. This poor response in LP which is mainly a T-lymphocyte-mediated disease could be explained by its predominant action on neutrophils rather than lymphocytes.25 The only disadvantage of NB-UVB is the frequent hospital visits.
Limitations
A larger sample size and longer-term follow-up are required. Only 3 treatment options were compared in this study but many more options have been used for lichen planus.
Conclusion
NB-UVB was found to be the best steroid-sparing treatment. Azathioprine was better in achieving faster response and longer remission. Dapsone showed a poor response in terms of disease control and side effects. Treatment choice must depend upon the feasibility of frequent visits to the hospital and the presence of co-morbidities.
Ethical approval
The study was approved by the Institutional Review Board at Government Stanley Medical College, number 21/12/2017, dated 21/12/2017.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript, and no images were manipulated using AI.
References
- A study of depression and quality of life in patients of lichen planus. Sci World J. 2015;817481
- [CrossRef] [PubMed] [Google Scholar]
- Treatments for cutaneous lichen planus: A systematic review and meta-analysis. Am J Clin Dermatol. 2016;17:11-22.
- [CrossRef] [PubMed] [Google Scholar]
- Psychobiological aspects of patients with lichen planus. Curr Psychiatr.. 2009;16:370-80.
- [Google Scholar]
- Treatment of severe lichen planus with mycophenolate mofetil. J Am Acad Dermatol. 2003;49:1063-66.
- [CrossRef] [PubMed] [Google Scholar]
- Successful treatment of resistant hypertrophic and bullous lichen planus with mycophenolate mofetil. Arch Dermatol. 1999;132:1420-1.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment of lichen planus and lichen nitidus with itraconazole: A report of six cases. Cutis. 1998;62:247-8.
- [Google Scholar]
- Lichen planus: Epidemiology, course, therapy in 580 patients. Dermatology. 1996;198:193161.
- [Google Scholar]
- Treatment of lichen planus with a short course of oral prednisolone. Br J Dermatol. 1990;123:550-1.
- [CrossRef] [PubMed] [Google Scholar]
- Erosive and generalized lichen planus responsive to azathioprine. Clin Exp Dermatol. 1996;21:56-7.
- [PubMed] [Google Scholar]
- Dapsone versus corticosteroids in lichen planus. Indian J Dermatol Venereol Leprol. 1999;65:66-8.
- [PubMed] [Google Scholar]
- Ultraviolet-B treatment for cutaneous lichen planus. Photodermatol Photoimmunol Photomed. 2008;24:83-6.
- [CrossRef] [PubMed] [Google Scholar]
- The clinical features, malignancy potential and systemic associations of oral lichen planus: A study of 723 patients. J Am Acad Dermatol.. 2002;46:207-14.
- [CrossRef] [PubMed] [Google Scholar]
- Familial mucosal lichen planus in three successive generations. Int J Dermatol.. 2005;44:81-2.
- [CrossRef] [PubMed] [Google Scholar]
- Familial oral lichen planus: Presentation of six families. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102:12-15.
- [CrossRef] [PubMed] [Google Scholar]
- 12-item pruritus severity scale: Development and validation of new itch severity questionnaire. Biomed Res International. 2017;3896423
- [CrossRef] [PubMed] [Google Scholar]
- Treatment of lichen planus with a short course of oral prednisolone. Br J Dermatol. 1990;123:550-1.
- [CrossRef] [PubMed] [Google Scholar]
- Azathioprine for the treatment of severe erosive oral and generalized lichen planus. Acta Derm Venereol. 2001;81:378-9.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy of dapsone in lichen planus. Indian J Dermatol Venereol Leprol.. 1985;51:115.
- [Google Scholar]
- Efficacy of steroidal vs non-steroidal agents in oral lichen planus: A randomised, open-label study. J Laryngol Otol. 2017;131:69-76.
- [CrossRef] [PubMed] [Google Scholar]
- Cutaneous and mucosal lichen planus: A comprehensive review of clinical subtypes, risk factors, diagnosis and prognosis. Sci World J. 2014;2014:742826.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of the narrow band UVB versus systemic corticosteroids in the treatment of lichen planus: A randomized clinical trial. J Res Med Sci. 2011;16:1578-82.
- [PubMed] [PubMed Central] [Google Scholar]
- Dapsone in the treatment of erosive lichen planus. J Am Acad Dermatol. 1985;12:567-70.
- [CrossRef] [PubMed] [Google Scholar]






