Translate this page into:
Comparative study on the efficacy and safety of bepotastine besilate versus levocetirizine in chronic spontaneous urticaria: A randomised, open-label, parallel study
Corresponding author: Kumaravelu Punnagai, Department of Pharmacology, Sri Ramachandra Medical College & Research Institute, Chennai, Tamil Nadu, India. kpunnagaimd@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Gayathri E, Sowmya P, Punnagai K, Mahalakshmi V. Comparative study on the efficacy and safety of bepotastine besilate versus levocetirizine in chronic spontaneous urticaria: A randomised, open-label, parallel study. Indian J Dermatol Venereol Leprol 2023;89:671-9
Abstract
Background
Urticaria is a common skin disease which often causes impairment in the quality of life. The ideal drug for chronic urticaria would have antihistaminic and anti-inflammatory actions. Bepotastine besilate is a recently approved novel anti-allergic agent with multiple mechanisms of action; levocetirizine is a potent and selective second-generation H1 receptor antagonist used in the treatment of urticaria.
Aim
To compare the efficacy and safety of bepotastine besilate versus levocetirizine in patients with chronic spontaneous urticaria.
Methods
The study design is a randomised, open-label, parallel-group, prospective interventional study. The study subjects were randomly assigned to either of the two groups a and b, each group had 50 patients with chronic urticaria. Statistical analyses were performed using (SPSS, version 18) for all the variables. Chi-square test was used for comparison between categorical variables. An unpaired student’s t-test was done for quantitative variables.
Results
There was a significant decrease in mean urticaria activity score (P < 0.001), chronic urticaria quality of life (P < 0.001) and clinical global improvement (P < 0.001) in both the treatment groups but this improvement was higher in the bepotastine than in the levocetirizine group. There was no significant difference in the mean of absolute eosinophil count, C-reactive protein, aspartate transaminase, alanine transaminase from baseline to 4th week between the two study groups. Visual analogue scale showed statistically significant improvement from baseline to 4th week (P < 0.001) of follow-up but this increase was higher in levocetirizine group (0.64-4.24) than in bepotastine group (0.56-2.56)
Limitations
Blinding was not done. To assess the efficacy and safety of bepotastine, a larger study can be planned.
Conclusion
This study found that bepotastine is superior to levocetirizine and showed a statistically significant reduction in mean urticaria activity score 7, improved quality of life and clinical global improvement in patients with urticaria.
Keywords
Urticaria
bepotastine
levocetirizine
urticaria activity score 7
Plain Language Summary
Chronic spontaneous urticaria, defined by the persistence of wheals for at least 6 weeks, affects 15–20 % of the population once or more during lifetime. It can dramatically alter the quality of life, in particular, sleep and generates numerous consultations and hospitalization. This study was carried out at Sri Ramachandra Institute of Higher Education and Research. It aimed to find out the efficacy and safety of bepotastine besilate versus levocetirizine in patients with chronic spontaneous urticaria. The authors found that at the end of the fourth week, there was significantly more reduction in the appearance of wheals, itching, daytime sedation and improvement in the quality of life in patients treated with bepotastine when compared with levocetirizine. Bepotastine is, thus, more efficacious and well-tolerated.
Introduction
Urticaria is one of the most common skin diseases causing redness, and swelling in the dermis and epidermis layers and are severely pruritic. Urticaria is defined as ‘acute’ if it lasts for less than six weeks and ‘chronic’ if it lasts for more than six weeks.1,2 It affects 15-20% of the population once or more during lifetime. The worldwide incidence is 0.1-3% of the population women being affected twice more as men.2,3
Chronic urticaria is further sub-divided into chronic spontaneous urticaria and chronic inducible urticaria. International guidelines recommend non-sedating antihistamines once daily as first-line therapy for chronic spontaneous urticaria and chronic inducible urticaria.1,4,5
Newer treatments are being developed, but antihistamines remain the cornerstone of the therapeutic approach.6-8 Second-generation H1-antihistamines, compared with their first-generation drugs, have demonstrated improved peripheral H1-receptor selectivity, decreased lipophilicity and additional antiallergic properties apart from being histamine inverse agonists.9 The mast cell is the major effector cell in most forms of urticaria. Allergies are mediated through immunoglobulin E signalling which triggers mast cell degranulation. Histamine plays a major role in the activation of mast cells which leads to the development of erythema, itching and wheels. Urticaria is characterised by an inflammatory infiltrate comprising CD4, CD8, T lymphocytes, eosinophils, basophils and neutrophils. So, the ideal drug should have anti-inflammatory action. Bepotastine besilate is a recently approved novel antiallergic agent with multiple mechanisms of action.10,11 It is a second-generation H1 receptor antagonist with mast cell stabilising effects.11,12 The anti-inflammatory actions of bepotastine besilate include inhibition of leukotriene B4 and attenuating eosinophil chemotaxis and activation.13-18 Bepotastine also inhibits the biosynthesis of proinflammatory cytokine production by keratinocytes, including inhibition of CD54 expression. Bepotastine, along with several other H1 antihistamines, reduces vascular hyperpermeability in both antigen-induced and histamine-induced hyperpermeability models.19,20 It is approved in Japan, India and USA for the treatment of various diseases like allergic conjunctivitis, atopic dermatitis and urticaria.21-29 Bepotastine also inhibits histamine-induced wheal and flare response in vivo.30
Levocetirizine is the active enantiomer of cetirizine. It is a potent and selective second-generation H1 receptor antagonist and is used in the treatment of allergic rhinitis and urticaria with fewer side effects.31 Compared with cetirizine, it has twice the affinity for the histamine H1- receptor, low volume of distribution and non-renal clearance, and less brain penetration. These favourable features may be caused by levocetirizine’s pharmacokinetic and pharmacodynamic properties including high bioavailability, low volume of distribution, high potency and H1-receptor occupancy.31 Hence this study’s aim was to compare the efficacy and safety of bepotastine besilate versus levocetirizine in patients with chronic spontaneous urticaria. Levocetirizine is a potent second-generation H1 receptor antagonist with fast onset, long duration of action, with well-tolerated adverse effect profile when compared with other second-generation antihistamines.31 The primary objective is to compare the efficacy of improvement in the intensity of itching, wheals and the secondary objective is to assess the safety, overall improvement and decrease in the severity of attacks in patients with chronic urticaria treated with bepotastine and levocetirizine.
Materials and methods
This study is a randomised, open-label, parallel-group, prospective interventional study conducted in the department of dermatology, Sri Ramachandra Institute of Higher Education and Research, Porur, Chennai. The study was approved by Institutional Ethics Committee ref: IEC/17/AUG/135/32 and voluntary informed written consent was obtained from participants after explaining the risk and benefits to the patient. The study was conducted as per the International Council of Harmonization-Good Clinical Practice guidelines and the ethical guidelines for biomedical research on human participants by ICMR (2017). The clinical trial registration number is CTRI/2017/10/010232. The study subjects were randomly assigned using a computer-generated randomisation chart to either of the two groups a and b, each group consists of 50 patients with chronic urticaria. The patients diagnosed as chronic spontaneous urticaria by the physician met the criteria for urticaria as defined by the EAACI/GA2LEN/EDF/WAO criteria [European Academy of Allergy and Clinical Immunology/Global Allergy And Asthma European Network /European Dermatology Forum /World Allergy Organization WAO-Urticaria Diagnostic Criteria] were included in the study. The inclusion and exclusion criteria are mentioned in Table 1. Withdrawal criteria included serious adverse events which warrant withdrawal of the participant. A history of drug allergy was obtained at baseline. Any adverse event that occurred was reported to the principal investigator immediately and appropriate steps were taken to treat the existing adverse events in that patient. The drug used in the study was prescribed accordingly and adverse effects were monitored accordingly. The patients who did not respond to the study drugs were given the rescue drug, the tablet chlorpheniramine maleate 4 mg.
| Inclusion criteria | Exclusion criteria |
|---|---|
| Adult patients (18-65 years) of both sexes. (male and female) | Patients with any dermatological condition that could interfere with the efficacy evaluation (including eczema, contact dermatitis, atopic dermatitis, nummular eczema, asteatosis eczema, angioedema, urticaria pigmentosa, psoriasis or ichthyosis, autoimmune disorders, Hodgkin disease, |
| Symptom score of ≥10 (i.e., moderate to severe intensity UAS7 scores) during the baseline visit | Known hypersensitivity to antihistamine, |
| Any clinically significant condition (cardiovascular, neurological, hepatic, renal or malignant diseases) | |
| Patients who received UV light therapy before entry, | |
| Patients who had received antihistamines (including H2 receptor antagonists) within 3 days, Non-steroidal anti-inflammatory drugs within 3 days, topical or systemic steroids within 4 weeks, Astemizole within 6 weeks, ketotifen within 2 weeks, anti-Leukotrienes within 3 days, | |
| Pregnant and lactating women. | |
| Subject who was enrolled in another investigational drug study during the same period |
UAS: Urticaria activity score, H2: Histamine, UV: Ultraviolent
Efficacy assessments
Primary endpoints were measured through urticaria activity score 7 (UAS7)-number of wheals and intensity of itching each on a 0-3 scale each day. Wheals were graded as follows 0- none, 1- mild (<20 wheals/24h), 2- moderate (21-50 wheals/24h) 3- intense (>50 wheals/24h) and itching was graded as follows 0- none, 1- mild, 2- moderate, 3- intense. The weekly urticaria activity score 7 ranged from 0 to 42.
Secondary endpoints were: (1) Change in chronic urticaria quality of life. It consists of 18 questions and each statement or question is scored on a 5-point scale at the baseline visit and at the end of the treatment. 1- not at all, 2- a little, 3- somewhat, 4- a lot and 5- very much.32 (2) Clinical global impression-global improvement scale compared to his/her condition at baseline, how much has he/she responded. 1- very much improved, 2- much improved, 3- minimally improved, 4- no change, 5- minimally worse, 6-much worse and 7-very much worse. (3) Chronic Urticaria Quality of life (CU-Q2OL) domains are: 1- Pruritus, 2- Wheals, 3- Eyes swelling, 4- Urticaria interferes with my work, 5- Urticaria interferes with my sleep, 6- Urticaria interferes with my spare time, 7- Urticaria interferes with my social relationship, 8- Do you have difficulties in falling asleep? 9- Do you wake up during the night?10- Do you feel tired during the day because of your bad night sleep? 11- Do you feel in a bad mood? 12- Do you have to put some limit in choosing your food? 13- Do you have to limit your physical activity? 14- Are you troubled by drugs side effects? 15- Are you embarrassed due to urticaria signs? 16- Are you embarrassed in going to public places? 17- Do you have any problems in using cosmetics? 18- I have some limits in choosing clothes material. (4) Absolute eosinophil count (5) C-reactive protein: It was evaluated during baseline and at the end of the study.
The safety assessments in both study groups were:
Liver function tests such as serum aspartate transaminase and serum alanine transaminase were assessed at the baseline and at the end of the study.
The visual analogue scale is used to assess the degree of daytime sedation between the groups at the baseline and at the end of the study.
Any other side effects associated with the treatment were noted in the diary provided for the patients.
The sample size for the study was calculated for comparing itching by using power and sample size calculator software (PS: Power and Sample Size Calculator 3.1.6 developed by D.Dupont and Plummer Jr. of Vanderbilt School of Medicine, USA) by considering the power of 80% and confidence interval of 95%, with the alpha value of 0.05. The expected sample proportions were 60% (p1) and 30% (p2). These parameters gave a sample size of 50 in each arm.
Demographic data were collected at baseline. Each patient underwent a complete baseline clinical examination before entering the study. All these details were recorded in the case report form and written informed consent was obtained from them duly. The baseline primary and secondary pre-treatment assessments along with biochemical parameters were also assessed. The treatment was continued for a period of 4 weeks by giving tab. bepotastine besilate (Lupin Pharmaceuticals limited) (20 mg/day, single tablet (10 mg) orally twice a day in the morning and night after food for study group A and study group B was given tab. levocetirizine (Dr. Reddy’s Laboratories) 5 mg single tablet orally once daily at night time after food. The tablets were given free of cost to the participants. The study participants were requested to record in a diary the above-mentioned secondary and other safety parameters. Adequate training was given to the patients in filling up these visual analogue scales and the adverse effects in the diary. Acute rescue medication (chlorpheniramine maleate 4 mg) was prescribed to the patients who did not respond to both the study drugs. Tablet chlorpheniramine 4 mg is routinely used as a standard drug for urticaria patients whose sleep is disturbed at night by the symptoms of urticaria in the study site. Patients were also instructed to record the time of using rescue medications in the ‘urticaria diary’. They were also advised to report any serious adverse effects immediately to the research team by phone. They were advised to bring back the urticaria diary and the empty tablet strip during each visit. The patients were also followed up by phone calls every seven days and were asked the above questions for wheals and Itching assessment and the details were recorded. At the end of 2nd week primary, and secondary endpoints and the adverse effects were assessed and at the end of four weeks, the study participants were assessed for UAS7 scores, chronic urticaria quality of life, the severity of daytime sedation using visual analogue scores, levels of c-reactive protein, absolute eosinophil count, aspartate transaminase, alanine transaminase and clinical global improvement score and associated occurrence of adverse drug reactions. The summary of trial procedures is mentioned in Table 2.
| Procedures | Baseline | 1st week | 2nd week | 3rd week | 4th week |
|---|---|---|---|---|---|
| Informed consent | ✓ | ||||
| Selection and randomisation | ✓ | ||||
| Demographic profile | ✓ | ||||
| Medical history | ✓ | ||||
| General and physical examination | ✓ | ✓ | ✓ | ✓ | ✓ |
| The signing of informed consent | ✓ | ||||
| UAS7 score, CGI-I Score | ✓ | ✓ | ✓ | ✓ | ✓ |
| CU-Q2oL (chronic urticaria quality of Life), AST, ALT, AEC | ✓ | ✓ | ✓ | ✓ | ✓ |
| Issue of trial medication | ✓ | ✓ | ✓ | ✓ | ✓ |
| Issue of UAS7 scale, CU-Q2oL, issue of VAS scale & CGI scale | ✓ | ||||
| Collection of completed UAS7 score, CU-Q2oL, VAS scale & CGI scale | ✓ | ✓ | ✓ | ✓ | |
| Efficacy assessments | ✓ | ✓ | ✓ | ✓ | |
| Adverse events assessments | ✓ | ✓ | ✓ | ✓ |
UAS: Urticaria activity score, CGI-I: Clinical global impression-improvement score, CU-Q2oL: Chronic urticaria quality of life questionnaire, AST: Aspartate transaminase, ALT: Alanine transaminase, AEC: Absolute eosinophil count, VAS: Visual analogue scale
Statistical analysis
Primary analyses involved all patients who were assigned the treatment. Chi-square test was used for comparison between categorical variables. Unpaired t-test was done for quantitative variables. These tests were used to determine the significant difference between the two groups. ‘P’ value of <0.05 was considered to be statistically significant. Statistical analyses were done for all the variables and performed using the statistical package for the social sciences (SPSS, version 18) for Microsoft Windows.
Results
A total of 115 patients who were diagnosed to have urticaria were screened for the study. Out of 115 patients, 100 were included in the study and the rest 15 patients were excluded as they were not meeting the eligibility criteria. The enrolled patients were randomised into the two treatment groups. There were no dropouts in the study. The flow chart for the study is given in Figure 1. The descriptive statistics of the age, and gender are analysed by chi-square test and the body mass index (BMI) of the patients is analysed by the unpaired t-test and the mean age, BMI and gender at baseline have been similar for the two groups of the patients [Table 3].
- Flow chart for Subject randomisation and outcome measures
| Baseline characteristics |
Bepotastine group N = 50 |
Levocetirizine group N = 50 |
P-value (P > 0.05) |
|||
|---|---|---|---|---|---|---|
| No. | % | No. | % | |||
| Gender | Male | 21 | 42.0 | 17 | 36.0 | 0.378 |
| Female | 38 | 76.0 | 33 | 66.0 | ||
| Total | 50 | 100.0 | 50 | 100.0 | ||
| Age (Mean ± SD) | 39.66 ± 12.70 | 37.04 ± 12.59 | 0.303 | |||
| Height | 158.50 ± 7.76 | 159.52 ± 7.3 | 0.190 | |||
| Weight | 69.28 ± 9.9 | 71.2 ± 9.9 | 0.334 | |||
| BMI | 27.77 ± 4.90 | 29.01 ± 4.98 | 0.212 | |||
| UAS7 | 22.3 ± 8.33 | 23.04 ± 6.35 | 0.486 | |||
| AEC | 350.82 ± 132 | 385.64 ± 138.93 | 0.274 | |||
Primary analyses
The primary analyses were done using an unpaired t-test for UAS7 score, CuQ-2oL scores, absolute eosinophil count, c-reactive protein levels, AST and ALT levels and visual analogue scale scores for severe daytime sedation. The chi-square test was used for the analysis of clinical global impression-global improvement scores and other adverse effects reported in the two treatment groups.
Urticaria activity score: The percentage of decrease in urticaria activity score 7(UAS7) from baseline to 2nd week in the bepotastine group was 77.27% and in the levocetirizine group was 65.2%. The P value was 0.021. At the end of 2nd week, the UAS7 scores were 82.4% in the bepotastine group and 72.6% in the levocetirizine group. The percentage decrease in UAS7 scores from baseline to end of 4th week in the bepotastine group was 86.3% and in the levocetirizine group was 73.8%. The P value was 0.001, which shows there was a significant statistical difference between the two treatment groups in the UAS7 score at the end of weeks 2, 3 and 4 of the treatment period [Table 4].
| UAS7 score (Wheal + Itching) |
Bepotastine group | Levocetirizine group | P-value |
|---|---|---|---|
| Mean ± SD | Mean ± SD | ||
| Baseline | 22.36 ± 8.33 | 23.04 ± 6.357 | 0.486 |
| 1st week | 7.92 ± 3.691 | 10.08 ± 7.12 | 0.337 |
| 2nd week | 5.16 ± 3.655 | 8.00 ± 6.234 | 0.021* |
| 3rd week | 3.94 ± 2.676 | 6.30 ± 4.441 | 0.009* |
| 4th week | 3.28 ± 2.348 | 6.02 ± 4.382 | 0.001* |
Analysis of secondary endpoints
Chronic urticaria quality of life questionnaire: the percentage of improvement in CU-Q2oL score from baseline to 4th week in the bepotastine group was 68.22% and in the levocetirizine group was 40.13%. There was a significant statistical difference between the two treatment groups in relation to the quality of life with the “P” value of 0.000 [Figure 2].
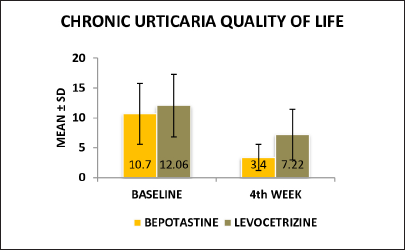
- Chronic urticaria quality (CU-Q2oL), of life at baseline and 4th week in between bepotastine and levocetirizine group
Clinical global impression-improvement scale assessment: chi-square test was used to analyse the percentage of improvement in the clinical global impression-improvement scale assessment score in both the treatment groups and the score from baseline to 2nd week was 61.7% in the bepotastine group and 38.5% in the levocetirizine group. In this analysis, the two treatment groups showed a significant statistical difference with a P value of 0.001. By the end of 4th week, the clinical global impression improvement was 77.3% in the bepotastine group, whereas in the levocetirizine group the percentage of improvement was 41% [Table 6]. Two groups were statistically significant in the 4th week also with the “P” value of 0.000
| Chronic urticaria - quality of life score (CU-Q2oL) | Bepotastine group Mean ± SD |
Levocetirizine group Mean ± SD |
P-value |
|---|---|---|---|
| Baseline | 10.70 ± 5.108 | 12.06 ± 5.235 | 0.188 |
| 4th week | 3.40 ± 2.195 | 7.22 ± 4.239 | 0.000* |
| Clinical global impression-improvement score (CGI-I) | Bepotastine group Mean ± SD |
Levocetirizine group Mean ± SD |
P-value |
|---|---|---|---|
| Baseline | 5.60 ± 0.728 | 5.66 ± 0.798 | 0.792 |
| 2nd week | 2.14 ± 1.050 | 3.48 ± 2.013 | 0.001* |
| 4th week | 1.92 ± 1.243 | 3.34 ± 2.026 | 0.000* |
The percentage of decrease in the mean absolute eosinophil count from baseline to 4th week in the bepotastine group was 3.2% whereas in the levocetirizine group was 2.1%. The percentage of decrease in the mean c-reactive protein from baseline to 4th week in the bepotastine group was 5.8% whereas in the levocetirizine group was 5.9%. The percentage of decrease in the mean levels of aspartate transaminase from baseline to 4th week in the bepotastine group was 1.5% whereas in the levocetirizine group was 6.9 %. The percentage of decrease in the mean levels of alanine transaminase from the baseline to 4th week in the bepotastine group was 0.5%, whereas in the levocetirizine group was 2.5%. In the pre-treatment period and at the end of 4th week there was no significant statistical difference between the two treatment groups in relation to the assessment of absolute eosinophil count, and C-reactive protein levels.
Safety assessments
There was no statistical significance between the bepotastine and the levocetirizine groups in the pre-treatment baseline measurement and also at the end of 4th week of the treatment period with regard to the increase in the liver enzymes aspartate transaminase and alanine transaminase. At the end of the treatment period, there was a significant statistical difference between the two treatment groups in relation to the severity of daytime sedation using a visual analogue scale. P value was 0.001.
There was a statistically significant increase in the occurrence of headaches in the levocetirizine group compared to the bepotastine group. Other adverse effects were dry mouth, nausea, vomiting and sore throat which were not statistically significant between the treatment groups. In the levocetirizine group, 11 patients did not respond. The investigators added the rescue medication tab. chlorpheniramine 4 mg at night to the patients who didn’t respond to the study drugs in both the treatment groups. In the bepotastine besilate group, eight patients did not respond and tab. chlorpheniramine 4mg at night was added, following which patients responded completely.
Discussion
Chronic urticaria (CU) is a relatively common chronic skin condition, which has a profound effect on the quality of life of those suffering from it. Hence, the primary goal of treatment should be directed towards ensuring a reduction in the disease symptoms and a decent quality of life.
This study was done to compare the efficacy and safety of bepotastine besilate, a newer histamine H1 receptor antagonist, with a standard drug levocetirizine, an H1 antagonist which is commonly prescribed for urticaria. The following parameters were evaluated for efficacy: urticaria activity score, chronic urticaria quality of life, and overall improvement by clinical global impression-global improvement scale. Safety was analysed with the visual analogue scale in terms of sedation and patient-reported adverse effects of drugs.
There was a significant decrease in mean urticaria activity score 7 (P < 0.001), chronic urticaria quality of life (P < 0.001), clinical global improvement (P < 0.001) in both the treatment groups. But this improvement was higher in the bepotastine than in levocetirizine group and no significant difference in the mean absolute eosinophil count (AEC), c-reactive protein (CRP), aspartate transaminase (AST) and alanine transaminase (ALT) from baseline to 4th week was seen between the two study groups. The difference in the mean scores of urticaria activity score between the treatment groups of bepotastine and levocetirizine was statistically significant from baseline to 2nd, 3rd and 4th week in both the treatment groups (P < 0.001) [Table 4], more in favour of the bepotastine group than the levocetirizine group. This could be due to multiple modes of action of bepotastine besiliate, such as inhibition of leukotriene B4, histamine and eosinophil chemotaxis. Bepotastine also is a mast cell stabilizer and has shown anti-inflammatory activity, thus reducing the severity of urticaria activity score 7 more than levocetirizine.
In a study by Takahashi et al. (2004), olapatadine and bepotastine showed a similar inhibitory effect on a flare but cetirizine showed marked inhibition of flare response at 2h and the effect was continued up to 24h.30 In another study done by Nettis et al. in 2006, levocetirizine was statistically superior to placebo in reducing mean scores for pruritus throughout the trial (P < 0.05) with 85% reduction in pruritus severity at the end of the active treatment.33
The difference in the mean scores of chronic urticaria quality of life was statistically significant and analysed using an unpaired t-test at the 4th week of follow-up (P = 0.001) value is applicable for the statistically difference between the levocetirizine and bepotastine groups at the end of 4th week but this improvement was higher in the bepotastine group than in the levocetirizine group [Figure 2]. The difference in mean scores of clinical global impressions-improvement scale in the bepotastine and the levocetirizine groups, analysed using the chi-square test, was statistically significant in 2nd week (P = 0.001) of follow-up and at end of the study at the 4th week (P < 0.001), but this improvement was higher in the bepotastine than in the levocetirizine group [Figure 3].
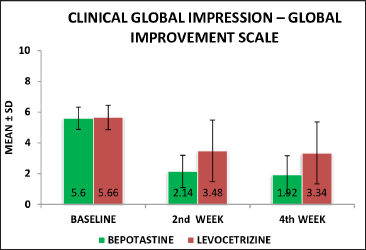
- Clinical global impression-improvement level at baseline, 2nd week & 4th week between the bepotastine and levocetirizine groups
When compared between the two study groups, we found there was no statistically significant difference in the mean scores of absolute eosinophil count, c-reactive protein, aspartate transaminase and alanine transaminase at baseline and at 4th week. In the present study, a higher number of participants in the levocetirizine group reported dry mouth, nausea /vomiting and sore throat than in the bepotastine group. However, the difference was not significant. Headache was significantly higher in the levocetirizine group (32%) than in the bepotastine group (12%) (P = 0.028).
The previous studies done with the study drug bepotastine did not include the assessment of the urticaria attacks in terms of urticaria activity score (wheals + itching); hence we have assessed urticaria activity score 7 and included other assessment scales like chronic urticaria quality of life, global improvement scale and sedation scale to assess various aspects of urticaria severity, recurrence and side effects. When the differences between the first and last follow-up visits were compared statistically, there was a significant reduction in the mean urticaria activity score [Table 4] and a significant improvement in the chronic urticaria quality of life [Table 5] and the overall global improvement scale [Table 6] was more in the bepotastine group when compared with levocetirizine. In our study, there was a statistically significant increase in the daytime sedation severity assessed by the mean visual analogue scale scores from baseline to 4th week in both the bepotastine and levocetirizine groups, but this reported increase in sedation was higher with levocetirizine [Table 7]. Bepotastine was well tolerated with respect to a reduction in the severity of daytime sedation and headache [Table 8]. The rationale for better tolerability and safety of bepotastine besilate is due to its selectivity for H1 receptors and non-binding to serotoninergic, muscarinic, beta-adrenergic and benzodiazepine receptors.
| Visual analogue scale (Severity of daytime sedation) |
Bepotastine group Mean ± SD |
Levocetirizine group Mean ± SD |
P-value |
|---|---|---|---|
| Baseline | 0.56 ± .907 | 0.64 ± 0.942 | 0.664 |
| 2nd week | 3.8- ± 2.466 | 5.00 ± 2.531 | 0.018* |
| 4th week | 2.56- ± 2.215 | 4.24 ± 2.544 | 0.01* |
| Adverse effects | Bepotastine group | Levocetirizine group | P-value | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Dry mouth | 6 | 12.0 | 11 | 22.0 | 0.287 |
| Nausea/vomiting | 3 | 7.0 | 5 | 12.0 | 0.458 |
| Headache | 6 | 12.0 | 16 | 32.0 | 0.028* |
| Sore throat | 3 | 9.0 | 4 | 7.0 | 0.693 |
Limitations
Limitations of the study were its open-label study design and the short duration of the study due to feasibility issues. To eliminate bias, patients were followed up by a dermatologist who was not a part of the study. We minimised the interobserver variation by training the research team personnel. Further studies can be planned with increased sample size and with long-term follow-up to assess the efficacy, safety and tolerability of bepotastine and levocetirizine. Furthermore, the quality of efficacy assessments of antihistaminic drugs can be assessed on the specific biomarkers of chronic urticaria like d-dimer and matrix metalloproteinase-9 levels.
Conclusion
In the present study, the study investigators found that the bepotastine besilate group has shown statistically significant reduction in the severity of itching and a reduction in the number of wheals assessed by the urticaria activity 7 scores (UAS7), improved quality of life (CU-Q2oL) and clinical global impression-improvement scale in patients with urticaria when compared to the levocetirizine group. In addition to the superior efficacy, the bepotastine group of patients have shown statistically significant safety in reducing the occurrence of adverse effects like daytime sedation and headache than the group treated with levocetirizine.
Acknowledgement
The investigators are very thankful to the management of Sri Ramachandra Institute of Higher Education and Research and the faculty and Postgraduates of the Department of Dermatology for their support to conduct the study. We are grateful to all the study participants for their cooperation to conduct the study.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflict of interest
There are no conflicts of interest.
References
- EAACI/GA (2) LEN/EDF/WAO guideline: Definition, classification and diagnosis of urticaria. Allergy. 2009;64:1427-43.
- [CrossRef] [PubMed] [Google Scholar]
- An approach to the patient with urticaria. Clin Exp Immunol. 2008;153:151-61.
- [CrossRef] [PubMed] [Google Scholar]
- Chronic urticaria: Indian context-challenges and treatment options. Dermatol Res Pract. 2013;2013:651737.
- [CrossRef] [PubMed] [Google Scholar]
- Consensus statement on the management of urticaria. Indian J Dermatol. 2011;56:485-9.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment options for the relief of chronic idiopathic urticaria symptoms. South Med J. 2008;101:186-92.
- [CrossRef] [PubMed] [Google Scholar]
- Histamine, histamine receptors, and their role in immunomodulation: An updated systematic review. Open J Immunol. 2009;2:9-41.
- [CrossRef] [Google Scholar]
- The pharmacology and use of H1receptor antagonist drugs. N Engl J Med. 1994;330:1663-70.
- [CrossRef] [PubMed] [Google Scholar]
- Structure and classification of H1-antihistamines and overview of their activities. Clin Allergy Immunol. 2002;17:65-100.
- [PubMed] [Google Scholar]
- General pharmacology of betotastinebesilate (TAU-284), a novel antiallergic agent. Jpn Pharmacol Ther. 1997;25:907-24.
- [Google Scholar]
- Brain histamine H1 receptor occupancy of orally administered antihistamines, bepotastine and diphenhydramine, measured by PET with 11C-doxepin. Br J Clin Pharmacol. 2006;61:16-26.
- [CrossRef] [PubMed] [Google Scholar]
- Anti-allergic activity of betotastinebesilate (TAU-284), a new antiallergic drug. Nihon Yakurigaku Zasshi. 1997;110:19-29.
- [CrossRef] [PubMed] [Google Scholar]
- Inhibitory effect of betotastinebesilate on antigen-induced airway eosinophil infiltration and peripheral blood eosinophilia in mice. Arzneimittelforschung. 1997;47:954-58.
- [PubMed] [Google Scholar]
- Clinical pharmacological study of anti-allergic agent TAU-184(bepotastinebesilate) - the effect on counting of eosinophils in nasal discharge, and the patency improvement of nasal cavity. J Clin Ther Med. 1997;13:1401-12.
- [Google Scholar]
- Antiallergic action of betotastinebesilate (TAU-284) in animal models: A comparison with ketotifen. Pharmacology. 1998;57:206-14.
- [CrossRef] [PubMed] [Google Scholar]
- Inhibitory effect on anaphylactic reaction and histamine antagonizing action [in guinea pigs] of betotastinebesilate (TAU-284), a novel anti-allergic drug. Jpn Pharmacol Ther. 1997;25:879-88.
- [Google Scholar]
- Suppression effects of the novel drug bepotastinebesilate (TAU-284) on experimental asthmatic reactions in guinea pigs. Jpn Pharmacol Ther. 1997;25:889-94.
- [Google Scholar]
- Suppression by bepotastinebesilate of substance P-induced itch-associated responses through the inhibition of the leukotriene B4 action in mice. Eur J Pharmacol. 2006;547:59-64.
- [CrossRef] [PubMed] [Google Scholar]
- Down modulatory effects of the antihistaminic drug bepotastine on cytokine/chemokine production and CD54 expression in human keratinocytes. Skin Pharmacol Physiol. 2009;22:45-48.
- [CrossRef] [PubMed] [Google Scholar]
- Bepotastinebesilate, a highly selective histamine H1 receptor antagonist, suppresses vascular hyperpermeability and eosinophil recruitment in in vitro and in vivo experimental allergic conjunctivitis models. Exp Eye Res. 2010;91:85-91.
- [CrossRef] [PubMed] [Google Scholar]
- Early phase II study of TAU284(betotastinebesilate) on chronic urticaria. J Clin Ther Med. 1997;13:1199-215.
- [CrossRef] [Google Scholar]
- Late phase II study of TAU284(betotastinebesilate) on chronic urticaria - optimal dose finding study by double-blind technique. J Clin Ther Med. 1997;13:1237-57.
- [CrossRef] [Google Scholar]
- Clinical evaluation of TAU284(betotastinebesilate) on eczema/dermatitis, prurigo, and pruritus cutaneus. J Clin Ther Med. 1997;13:1383-400.
- [Google Scholar]
- Investigation of the clinical effects and safety of bepotastinebesilate (Talion tablets) on patients with chronic hives. Prog Med. 2004;24:151-5.
- [Google Scholar]
- Investigation of effectiveness and safety of bepotastinebesilate (Talion tablets) in cedar pollen allergy. Prog Med. 2002;22:2472-7.
- [Google Scholar]
- Efficacy and safety investigation of bepotastinebesilate (Talion tablets) in patients with atopic dermatitis. J New Rem Clin. 2005;54:1325-31.
- [Google Scholar]
- The effect of prophylactic treatment with bepotastine in patients with Japanese cedar pollinosis. Practica Otologica. 2002;95:531-7.
- [Google Scholar]
- Early phase II study of TAU-284(betotastinebesilate) on perennial allergic rhinitis. J Clin Ther Med. 1997;13:1217-35.
- [Google Scholar]
- Late phase II clinical study of TAU284 for perennial allergic rhinitis - dose finding study by the doubleblind method. J Clin Ther Med. 1997;13:1259-86.
- [Google Scholar]
- Effects of bepotastine, cetirizine, fexofenadine, and olopatadine on histamineinduced wheal-and flare-response, sedation, and psychomotor performance. Clin Exp Dermatol. 2004;29:526-32.
- [CrossRef] [PubMed] [Google Scholar]
- Levocetirizine: The latest treatment option for allergic rhinitis and chronic idiopathic urticaria. Allergy Asthma Proc. 2007;28:724-34.
- [CrossRef] [PubMed] [Google Scholar]
- A new tool to evaluate the impact of chronic urticaria on quality of life: Chronic urticaria quality of life questionnaire (CU-Q2oL) Allergy. 2005;60:1073-8.
- [CrossRef] [PubMed] [Google Scholar]
- Levocetirizine in the treatment of chronic idiopathic urticaria: A randomized, double-blind, placebo-controlled study. Br J Dermatol. 2006;154:533-8.
- [CrossRef] [PubMed] [Google Scholar]






