Translate this page into:
Congenital cutis laxa with rectal and uterovaginal prolapse
2 Department of Dermatology, B. S. Medical College, Bankura, India
Correspondence Address:
Sanjiv V Choudhary
28, Modern Nagpur Society, Chhatrapati Nagar, Nagpur-15 [MS]
India
| How to cite this article: Choudhary SV, Bisati S, Koley S. Congenital cutis laxa with rectal and uterovaginal prolapse. Indian J Dermatol Venereol Leprol 2011;77:321-324 |
Abstract
A two-month-old female infant born of a consanguineous marriage, presented with loose, wrinkled and inelastic skin over the neck, axillae, trunk, inguinal region and thighs with slow elastic recoil. Patient also had systemic manifestations in the form of bilateral apical lobe consolidation of lung, bilateral inguinal hernia, rectal and uterovaginal prolapse. Histopathological examination of skin biopsy with special stain for elastic tissue revealed absence of dermal elastic tissue. Genital abnormalities in patients with congenital cutis laxa have been reported rarely. But rectal and uterovaginal prolapse have not been reported at an early age of two months. In the absence of mutational screening, with history and clinical findings our case is likely to be Type I autosomal recessive form of congenital cutis laxa.Introduction
Cutis laxa or generalized elastolysis is a heterogenous group of disorders characterized by loose and redundant skin with reduced elasticity. [1] Elastolysis is also seen involving internal body organs leading to various systemic manifestations. Cutis laxa can be congenital or acquired. Congenital cutis laxa can be autosomal dominant, autosomal recessive or X-linked. [2],[3],[4] The autosomal recessive forms are often associated with more severe multisystem involvement. The histopathology of the skin in a patient with cutis laxa reveals loss and/or fragmentation of elastic fibers. [5],[6] Autosomal dominant forms of congenital cutis laxa are of at least two types, caused by mutations in the elastin and fibulin-5 genes, respectively. Two previous studies have shown mutations in the elastin gene in autosomal dominant type of cutis laxa patients. [2],[6] Mutation in the gene encoding fibulin-5 can cause both autosomal dominant and autosomal recessive type of cutis laxa. [7] Mutations in fibulin-4 have also been discovered as a cause in autosomal recessive cutis laxa type I. [8] In autosomal recessive cutis laxa Type 2, mutations of ATP6VOA2 have been identified. [9] In autosomal recessive cutis laxa with progeroid features (wrinkly skin syndrome) the mutations have been identified in the PYCR1 gene. [10] The pathogenesis of cutis laxa involves disruption of the balance between the elastase inhibitor and elastase and also decreased synthesis of elastin by skin fibroblasts. [11]
We report a case of congenital cutis laxa with rectal and uterovaginal prolapse, noticed at very young age of two months.
Case Report
A two-month-old female infant born of consanguineous marriage was referred by a pediatrician for generalized lax skin since birth. The mother informed that the child had generalized lax skin since birth. One month after birth, she developed breathlessness for which she was admitted to the hospital. She was born at term with normal birth weight. The pregnancy was uneventful. She was the second child of the parents. There was no history of similar lax skin in any other family members. Her elder brother was normal. On general examination the child had an old premature look. On cutaneous examination the skin over the face, neck, axillae, trunk and thighs appeared to be wrinkled and thrown in loose folds giving an aged look [Figure - 1]. The skin could be easily stretched and showed slow recoiling.
 |
| Figure 1: Loose, wrinkled and inelastic skin involving the neck, trunk and thighs |
There was no laxity of the joints. On systemic examination the child appeared to be tachypneic with bilateral inguinal hernia. Examination of the gastrointestinal and genitourinary system revealed rectal and uterovaginal prolapse [Figure - 2] and [Figure - 3].
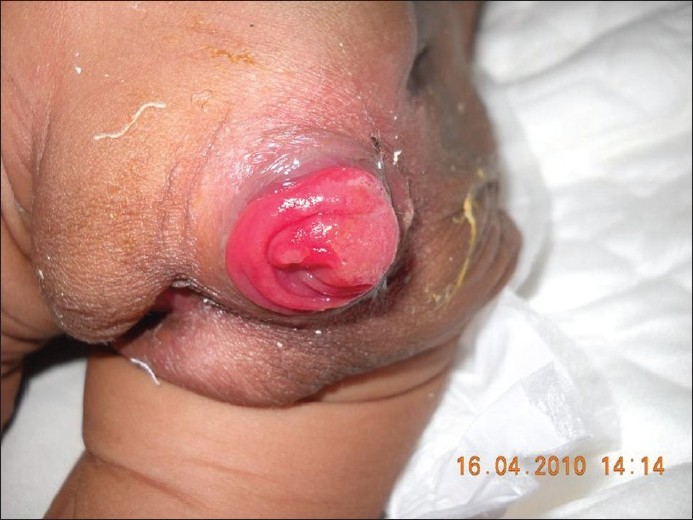 |
| Figure 2: Rectal prolapse |
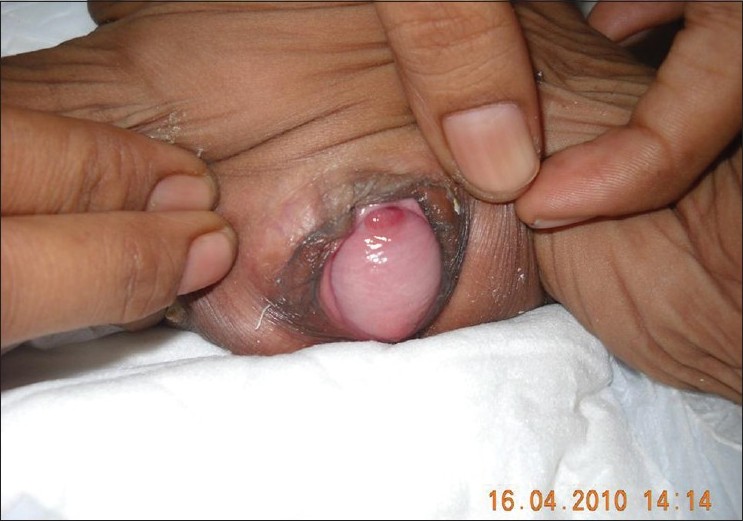 |
| Figure 3: Uterovaginal prolapse |
Skin biopsy with hematoxylin and eosin stain showed thinned out epidermis with mild hyperkeratosis and parakeratosis. The papillary dermis was unremarkable with nonspecific chronic inflammatory infiltrate. The deeper dermis showed mild collagenosis and unremarkable fibrous fatty tissue and adnexal structures. Special staining with Verhoeff Van Gieson′s elastic tissue stain was carried out which showed predominant collagen bundles [bright red] and adnexal structures [yellow]. The elastic tissue stain was conspicuously lacking [Figure - 4].
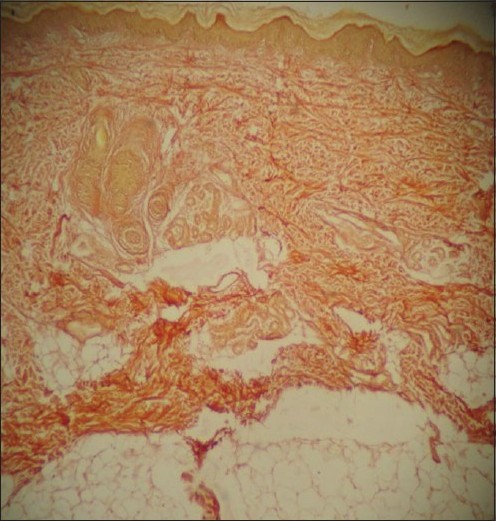 |
| Figure 4: Special staining with Verhoeff Van Gieson's elastic tissue stain revealing absence of dermal elastic fibers (×10) |
X-ray chest revealed consolidation of bilateral apical lobes of the lungs. Mutational screening was not done due to lack of the facility. On the basis of these clinical and histopathological findings, a diagnosis of congenital cutis laxa was made.
Discussion
Congenital onset is generally indicative of an autosomal recessive or X-linked form of cutis laxa. As a rule, the autosomal dominant form of cutis laxa has primarily skin involvement with few systemic complications and normal life expectancy and is thus largely a cosmetic problem with a good prognosis.
Type I autosomal recessive form of congenital cutis laxa is associated with severe multisystem complications that include emphysema, diaphragmatic hernia and gastrointestinal and genitourinary diverticula, cardiorespiratory complications and early death from corpulmonale. Hyperextensible skin with slow elastic recoil is the hallmark of cutis laxa. There are prominent skin folds around the knees, abdomen and thighs. [12] Systemic manifestations are also due to abnormalities in the internal elastic tissue.
In our case, history of consanguinity of marriage in the parents suggests a probable autosomal recessive mode of transmission. A classical cutaneous manifestation in the form of loose, wrinkled and inelastic skin with slow elastic recoil was present. Involvement of the respiratory system was present in the form of bilateral apical lobe pneumonia which could be due to aspiration. Our case also had gastrointestinal and genitourinary system involvement in the form of bilateral inguinal hernia, rectal prolapse and uterovaginal prolapse. Histopathological examination of skin biopsy with special elastic tissue staining revealed complete absence of dermal elastic tissue fibers. Type II autosomal recessive form of congenital cutis laxa is a less severe form and is characterized by growth retardation, developmental delay and ligamentous laxity. The X-linked recessive form of cutis laxa, formerly considered as Ehlers-Danlos Type IX, is now called the occipital horn syndrome. X-linked form of congenital cutis laxa is characterized by mild joint laxity, bladder diverticula, hernias and cranial occipital exostoses or horns. In the light of these findings, our patient was more likely to be a case of type I autosomal recessive form of congenital cutis laxa.
In the Ehlers-Danlos syndrome, the skin is hyperextensible but not lax and it recoils quickly. In pseudoxanthoma elasticum, the skin may be lax but it is associated with yellowish discoloration. It can be distinguished histologically by the presence of calcifications and other systemic manifestations.
In case of congenital cutis laxa, no treatment exists to prevent disease progression. Medical treatment is useful to control infection, to reduce morbidity and to prevent complications. The patient should be evaluated for internal organ involvement and should be treated for specific complications. Surgical correction of redundant skin folds, prolapses or hernias may be undertaken. However, surgery often produces only temporary benefit. A geneticist should be consulted for better management.
There are few reports of congenital cutis laxa from India. Sarkar et al., [13] presented an unusual family in which seven members were affected by the autosomal dominant variant of this disorder. Kothari et al., [14] reported congenital cutis laxa associated with multiple hernias and cardiac anomaly in an infant. Very recently an eight-year-old boy was reported with congenital cutis laxa with umbilical and paraumbilical hernias, emphysema and pulmonary artery branch stenosis. [15]
Genital abnormalities in patients with congenital cutis laxa have been rarely reported in world literature. There are only a few reports describing genital prolapse [16],[17] and rectal prolapse. [18] Rarity of literature on congenital cutis laxa has prompted us to present this interesting case of congenital cutis laxa with rectal and uterovaginal prolapse, presenting at a very young age of two months.
| 1. |
Pavithran K. Congenital cutis laxa. Indian J Dermatol Venereol Leprol 1992;58:217-8.
[Google Scholar]
|
| 2. |
Acharya KM, Mukhopadhyay AK, Jain R. Congenital cutis laxa. Indian J Dermatol Venereol Leprol 1996;62:314-5.
[Google Scholar]
|
| 3. |
Zhang MC, He L, Giro M, Yong SL, Tiller GE, Davidson JM. Cutis laxa arising from frameshift mutations in exon 30 of the elasting gene (ELN). J Biol Chem 1999;274:981-6.
[Google Scholar]
|
| 4. |
· Agha A, Sakati NO, Higginbottom MC, Jones KL Jr, Bay C, Nyhan WL. Two forms of cutis laxa presenting in the newborn. Acta Paediatr Scand 1978;67:775-80.
[Google Scholar]
|
| 5. |
Hashimoto K, Kanzaki T. Cutis laxa: Ultrastructural and biochemical studies. Arch Dermatol 1975;111:861-73.
[Google Scholar]
|
| 6. |
Kitano Y, Nishida K, Okada N, Mimaki T, Yabuuchi H. Cutis laxa with Ultrastructural abnormalities of elastic fiber. J Am Acad Dermatol 1989;21:378-80.
[Google Scholar]
|
| 7. |
Markova D, Zou Y, Ringpfeil F, Sasaki T, Kostka G, Timpl R, et al. Genetic heterogeneity of cutis laxa: A heterozygous tandem duplication within the fibulin-5 (FBLN5) gene. Am J Hum Genet 2003;72:998-1004.
[Google Scholar]
|
| 8. |
Fornieri C, Qualino D, Lungarella G, Cavarra E, Tiozzo R, Giro MG, et al. Elastin production and degradation in cutis laxa acquisita. J Invest Dermatol 1994;103:583-8.
[Google Scholar]
|
| 9. |
Kornak U, Reynders E, Dimopoulou A, van Reeuwijk J, Fischer B, Rajab A, et al. Impaired glycosylation and cutis laxa caused by mutation in the vesicular H+-ATPase subunit ATP6V0A2. Nat Genet 2008;40:32-4.
[Google Scholar]
|
| 10. |
Reversade B, Escande-Beillard N, Dimopoulou A, Fischer B, Chng SC, Li Y, et al. Mutations in PYCR1 cause cutis laxa with progeroid features. Nat Genet 2009;41:1016-21.
[Google Scholar]
|
| 11. |
Hucthagowder V, Sausgruber N, Kim KH, Angle B, Marmorstein LY, Urban Z. Fibulin-4: A novel gene for an autosomal recessive cutis laxa. Am J Hum Genet 2006;78:1075-80.
[Google Scholar]
|
| 12. |
Patton MA, Tolmie J, Ruthnum P, Bamforth S, Baraitser M, Pembrey M. Congenital cutis laxa with retardation of growth and development. J Med Genet 1987;24:556-61.
[Google Scholar]
|
| 13. |
Sarkar R, Kaur C, Kanwar AJ, Basu S. Cutis laxa in seven members of a north-Indian family. Pediatr Dermatol 2002;19:229-31.
[Google Scholar]
|
| 14. |
Kothari PR, Gupta A, Shankar G, Kulkarni BK, Dharap S. Infantile congenital cutis laxa with multiple hernias and ventricular septal defect. Indian J Surg 2005;67:43-4.
[Google Scholar]
|
| 15. |
Mauskar A, Shanbag P, Ahirrao V, Nagotkar L. Congenital cutis laxa. Ann Saudi Med 2010;30:167-9.
[Google Scholar]
|
| 16. |
Paladini D, Di Spiezio Sardo A, Mandato VD, Guerra G, Bifulco G, Mauriello S, et al. Association of cutis laxa and genital prolapse: A case report. Int Urogynecol J Pelvic Floor Dysfunct 2007;18:1367-70.
[Google Scholar]
|
| 17. |
Damkier A, Brandrup F, Starklint H. Cutis laxa: Autosomal dominant inheritance in five generations. Clin Genet 1991;39:321-9.
[Google Scholar]
|
| 18. |
Bechelli LM, Pagnano PM, De Souza NM, Soares LM, Rossi MA, Marin-Neto JA, et al. Generalized congenital cutis laxa associated with visceral lesions. Ann Dermatol Venereol 1985;112:835-9.
[Google Scholar]
|
Fulltext Views
4,839
PDF downloads
3,204





