Translate this page into:
Could co-infection with Anaplasma play a role in Borrelia-associated primary cutaneous marginal zone B-cell lymphomas?
2 Department of Experimental and Clinical Medicine, Institute of Dermatology, University of Udine, Udine, Italy
Correspondence Address:
Serena Bonin
Department of Medical Sciences, Unit of Dermatology, Cattinara Hospital, Strada di Fiume, 447, 34149 Trieste
Italy
| How to cite this article: Bonin S, Stinco G, Patriarca MM, Trevisini S, di Meo N, Trevisan G. Could co-infection with Anaplasma play a role in Borrelia-associated primary cutaneous marginal zone B-cell lymphomas?. Indian J Dermatol Venereol Leprol 2016;82:81-84 |
Sir,
Borrelia burgdorferi, the causative agent of Lyme disease, infects humans through Ixodes tick bites. In Europe, several Borrelia strains have been associated with primary cutaneous B-cell lymphoma, in particular, cutaneous marginal zone B-cell lymphoma.[1] It has already been reported that clinical regression of B. burgdorferi- associated cutaneous marginal zone B-cell lymphomas did not occur after specific antimicrobial therapy although polymerase chain reaction (PCR) analysis for Borrelia became negative.[2] Persistence of the lymphoma could be related to host factors or another unknown antigenic stimulation unresponsive to antimicrobial treatment. Significant co-infections in Lyme disease could be due to microorganisms transmitted by the same vector. In our province, (Trieste, North-Eastern Italy) co-infection of Borrelia with Anaplasma phagocytophilum and Rickettsia helvetica is rather frequent: 15 (3.5%) patients tested positive to Borrelia and A. phagocytophilum during 2013. To further investigate this association, we carried out a study investigating the presence of co-infections in patients with proven Borrelia-related cutaneous marginal zone B-cell lymphoma.
We report on four consecutive patients with the morphological features of primary cutaneous marginal zone B-cell lymphoma.
The first three patients participating in this study had previously been reported.[3] Patients were investigated for the presence of B. burgdorferi, A. phagocytophilum and R. helvetica with conventional serology and for Borrelia also by PCR.[3]
A 62-year-old man presented with cutaneous marginal zone B-cell lymphoma characterized by pink, non-tender, pruritic skin nodules on his right inner thigh [Figure - 1]. Biopsy showed a nodular lymphoid proliferation of small lymphocytes within the dermis. Clonality of B lymphocytes was revealed by Ki67 staining. Both serologic tests (ELISA IgG and Western blot) and PCR assays for B. burgdorferi were positive. Serologic analysis for IgG antibodies to A. phagocytophilum was positive while the test was negative for R. helvetica.
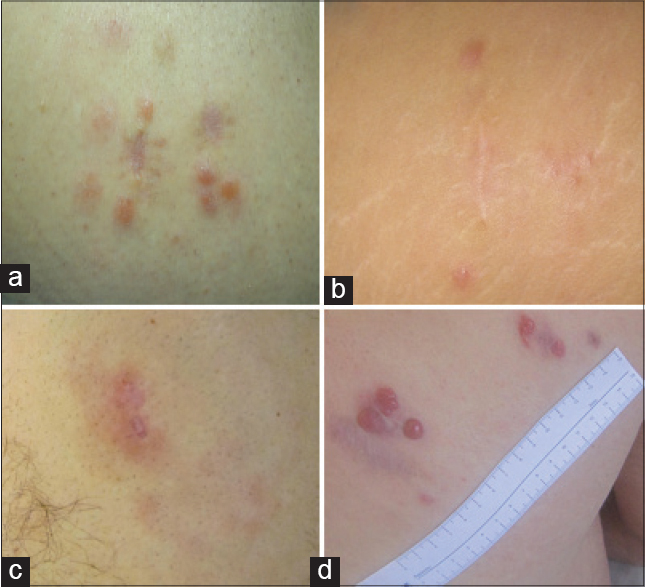 |
| Figure 1: Clinical manifestation of the lesions: (a) Patient 1: Pink, non-tender, skin nodules on the right inner thigh; (b) Patient 2: Erythematous, non-tender papules on the right leg; (c) Patient 3: Infi ltrated erythematous patch on the right leg; (d) Patient 4: Nodular erythematous lesion on the back |
A 46-year-old woman presented with five itchy erythematous, non-tender and painless papules on her right leg [Figure - 1]. Routine laboratory investigations were normal. Biopsy of the lesion showed a dense nodular infiltrate of lymphocytes separated from the epidermis by a Grenz zone [Figure - 2].
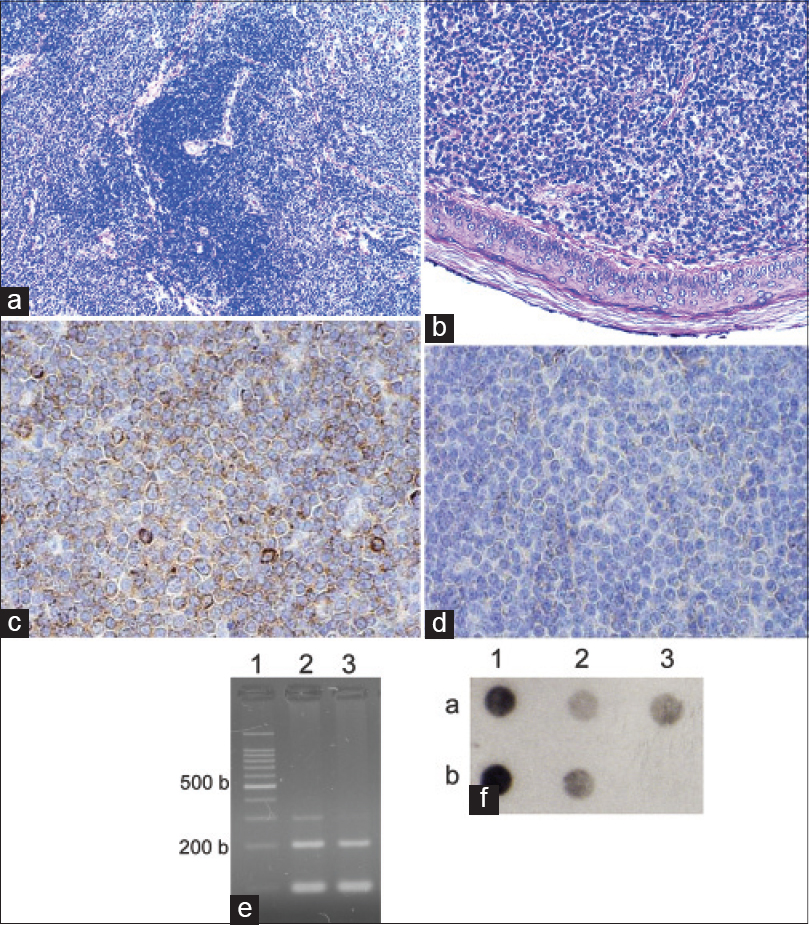 |
| Figure 2: Laboratory investigations of cases: Morphology, immunohistochemistry (IHC) detection of B-cell clonality (only Ki67 staining was undertaken in patient 1) and polymerase chain reaction (PCR) assays: (a) Dense nodule within the dermis (H and E, ×100) showing the characteristic arrangement of cells with central nodules of reactive lymphocytes surrounded by neoplastic cells; (b) Grenz zone separating nodular infi ltrate and epidermis (H and E, ×200); (c) IHC showing monoclonal Lambda Ig expression at the periphery of the nodular infi ltrate (×400, frozen section); (d) IHC showing inconspicuous kappa Ig expression at the periphery of nodular infi ltrate (×400, frozen section); (e) DNA extracts availability and quality controls (1: 100 base pair ladder; 2: DNA from peripheral blood; 3: DNA from formalin fi xed-paraffi n embedded biopsy); (f), typical dot blot of Flagellin PCR (a1, b1 and b2 positive controls of Borrelia afzelii, Garinii and sensu stricto, respectively; a2 PCR from peripheral blood; a3 PCR from formalin fi xed-paraffi n embedded biopsy; b3 negative control) |
B-cell clonality was assessed by lambda and kappa light chain immunohistochemistry in frozen sections [Figure - 2]. In view of these findings and the immunophenotype, a diagnosis of cutaneous marginal zone B-cell lymphoma was made.
Polymerase chain reaction assays for Borrelia DNA were positive both from biopsy and peripheral blood [Figure - 2]. Serological investigations for B. burgdorferi and R. helvetica were negative while A. phagocytophilum serology proved to be IgG positive.
A healthy 35-year-old man developed an infiltrated erythematous patch on his right leg [Figure - 1]. The patient had a previous history of Lyme disease.[3] Skin biopsy showed a top-heavy atypical lymphocytic infiltration involving the dermis and infiltrating the upper portion of subcutaneous fat. The characteristic Grenz zone of normal collagen was evident.
Polymerase chain reaction assays for Borrelia DNA were positive both from biopsy and peripheral blood. Serology for Borrelia was negative, but it was positive for A. phagocytophilum and R. helvetica.
A 36-year-old man developed a nodular erythematous lesion on his back [Figure - 1] which was excised and a diagnosis of marginal cell lymphoma was made. Later, five small nodular lesions appeared around the surgical scar. Results of routine laboratory examinations were normal. The biopsy showed a top-heavy atypical lymphocytic infiltration with a Grenz zone.
Polymerase chain reaction assays for Borrelia DNA were positive both from peripheral blood and biopsy. Serology for Borrelia and A. phagocytophilum was positive, but negative for R. helvetica.
All four patients underwent antibiotic treatment with doxycycline, 100 mg twice a day for 20 days. Lymphomas were treated in all patients with electrochemotherapy as already reported.[3] A complete response was obtained in all patients after 4 weeks. During the follow-up period, three patients relapsed as reported in [Table - 1].

The association between chronic infection with Borrelia and the development of cutaneous marginal zone B-cell lymphoma is conceptually and clinically similar to the one seen in B-cell lymphomas of the stomach, a proportion of which are associated with infection by Helicobacter pylori. Similarly, the suspected pathophysiology for the induction of lymphoma by Borrelia is chronic antigen-dependent immunostimulation triggering sustained lymphoid proliferation with oligoclonal and ultimately monoclonal B-cell selection.[4] It has been hypothesized that H. pylori eradication leads to the disappearance of intratumoral H. pylori specific T-cells and to lymphoma regression.[2] In the same way, complete regression of lymphoma lesions has been seen after antibiotic treatment for B. burgdorferi.[5]
Patients with Borrelia- related cutaneous marginal zone B-cell lymphoma unresponsive to antibiotic treatment may be subjected to other chronic antigen stimulation.[1] We focused on A. phagocytophilum and R. helvetica co-infections which are the most frequent microorganisms co-infected with B. burgdorferi by Ixodes ricinus in our area. We were unable to find any previous reports of the association between primary cutaneous B-cell lymphoma and other infections concurrent with Borrelia. Our data show that co-infections occur in some patients with Borrelia-related lymphomas. The impact of concurrent infections in Lyme disease has not yet been fully clarified because multiple infections are difficult to analyse as it is usually impossible to establish the infection that plays a predominant role in the pathological process due to frequent overlapping of clinical symptoms caused by borreliosis and co-infections. We speculate that in case of Borrelia- related primary cutaneous B-cell lymphoma, multiple infections may influence the immune system by decreasing co-surveillance and favoring a chronic B-cell stimulation towards an oligo/monoclonal B-cell proliferation. This may result in resistance to antibiotic therapy or in a persisting and self-maintaining disease driven by unknown antigenic stimulation even after the eradication of bacteria.
Our study had limitations due to lack of facility to detect A. phagocytophilum in tissue biopsy to demonstrate the co-infection and that Borrelia infection was not confirmed by serology in two patients. Nevertheless, to strengthen our data, three distinct PCR analyses have been carried out for Borrelia in both blood and biopsies for all patients. We were also limited by our small sample size and single geographic area. However, our study stresses on performing more comprehensive analyses in order to establish if A. phagocytophilum should always be included in the serologic testing of patients with a history of tick bite, in patients with Lyme disease and B. burgdorferi-related cutaneous marginal zone B-cell lymphoma since co-infection could be more frequent than so far suspected.
Acknowledgments
This study was partially supported by the FRA2012 grant from the University of Trieste. The authors would thank Dr. Diego Signoretto and Dr. Eugenio Leonardo who provided us with histological images and details of the cases and Dr. Daniela Casotto who gave us information about co-infections of Borrelia and A. phagocytophilum in our population during 2013. The authors are also grateful to Dr. Valentina Melita for the language revision of the manuscript.
Financial support and sponsorship
This study was partially supported by the FRA2012 grant from the University of Trieste.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Ponzoni M, Ferreri AJ, Mappa S, Pasini E, Govi S, Facchetti F, et al. Prevalence of Borrelia burgdorferi infection in a series of 98 primary cutaneous lymphomas. Oncologist 2011;16:1582-8.
[Google Scholar]
|
| 2. |
Du MQ. MALT lymphoma: Recent advances in aetiology and molecular genetics. J Clin Exp Hematop 2007;47:31-42.
[Google Scholar]
|
| 3. |
Gatti A, Stinco G, Trevisini S, di Meo N, Signoretto D, Leonardo E, et al. Electrochemotherapy as a novel treatment for primary cutaneous marginal zone B-cell lymphomas. Dermatol Ther 2014;27:244-7.
[Google Scholar]
|
| 4. |
Willemze R, Jaffe ES, Burg G, Cerroni L, Berti E, Swerdlow SH, et al. WHO-EORTC classification for cutaneous lymphomas. Blood 2005;105:3768-85.
[Google Scholar]
|
| 5. |
Sokol L, Naghashpour M, Glass LF. Primary cutaneous B-cell lymphomas: Recent advances in diagnosis and management. Cancer Control 2012;19:236-44.
[Google Scholar]
|
Fulltext Views
4,608
PDF downloads
2,860





