Translate this page into:
Crizotinib-induced oral lichenoid lesions
2 Department of Dermatovenereology, University Hospital of São João, Porto, Portugal
3 Department of Pneumology, Clinique Pasteur, Toulouse, France
4 Department of Oral Medicine, Claudius Regaud Institute and Cancer University Institute, Toulouse Oncopole, Toulouse, France
Correspondence Address:
Vincent Sibaud
Department of Oncodermatology and Oral Medicine, Claudius Regaud Institute and Cancer University Institute, Toulouse Oncopole
France
| How to cite this article: Sibaud V, Gomes NP, Raspaud C, Vigarios E. Crizotinib-induced oral lichenoid lesions. Indian J Dermatol Venereol Leprol 2020;86:209-211 |
Sir,
Crizotinib is a first-generation orally active anaplastic lymphoma kinase (ALK) inhibitor, also targeting mesenchymal-epithelial transition factor (MET) receptor and receptor of silencing 1 (ROS1) kinases, which is now United States Food and Drug Administration (FDA)- and European Medicines Agency (EMA)-approved for advanced ALK/ROS1-positive non-small cell lung cancer (NSCLC). It is associated with a favorable dermatologic safety profile, with low frequency of all-grade skin toxic effects (about 3%).[1] These dermatologic adverse events, however, have not been characterized until now. Ho and Chen recently described a patient treated for an NSCLC who developed a cutaneous lichenoid eruption induced by crizotinib.[2] We recently observed the development of a severe oral lichenoid reaction with crizotinib therapy.
A patient in her 60s was referred to the dermatology department for recurrent oral ulcerations. She did not report any history of skin disorders. She was treated for an advanced ALK-positive NSCLC with crizotinib for 7 months (500 mg/day). No other medication was reportedly prescribed. Two months after the initiation of treatment, she developed grade 2 stomatitis (according to the National Cancer Institute Common Terminology Criteria, version 4.0), with progressive occurrence of debilitating ulcers on the tongue and oral mucosa. The ulcers interfered with fluid/food intake and had a negative impact on her quality of life. Oral examination revealed a combination of ulcerative lesions with characteristic papular/plaque-like lesions and reticular white streaks involving both keratinized (dorsum of the tongue) and nonkeratinized mucosae (buccal mucosa, lateral aspects of the tongue, mucosal aspect of the lip) [Figure - 1]a and [Figure - 1]b. These oral mucosal changes had developed in an isolated manner, without any involvement of the skin, nails, or genital areas. A mucosal biopsy showed focal parakeratosis with hypergranulosis and a lichenoid interface dermatitis, combining a superficial band-like T-cell infiltrate in the upper lamina propria together with vacuolar basal changes [Figure - 2]. Symptomatic management was started with topical high-potency corticosteroids (0.05% clobetasol propionate cream – Dermoval®, twice a day), together with the promotion of optimal oral hygiene. After 1 month of therapy, the oral lesions had returned to grade 1, and the anticancer therapy was continued at the current dose [Figure - 3]a and [Figure - 3]b.
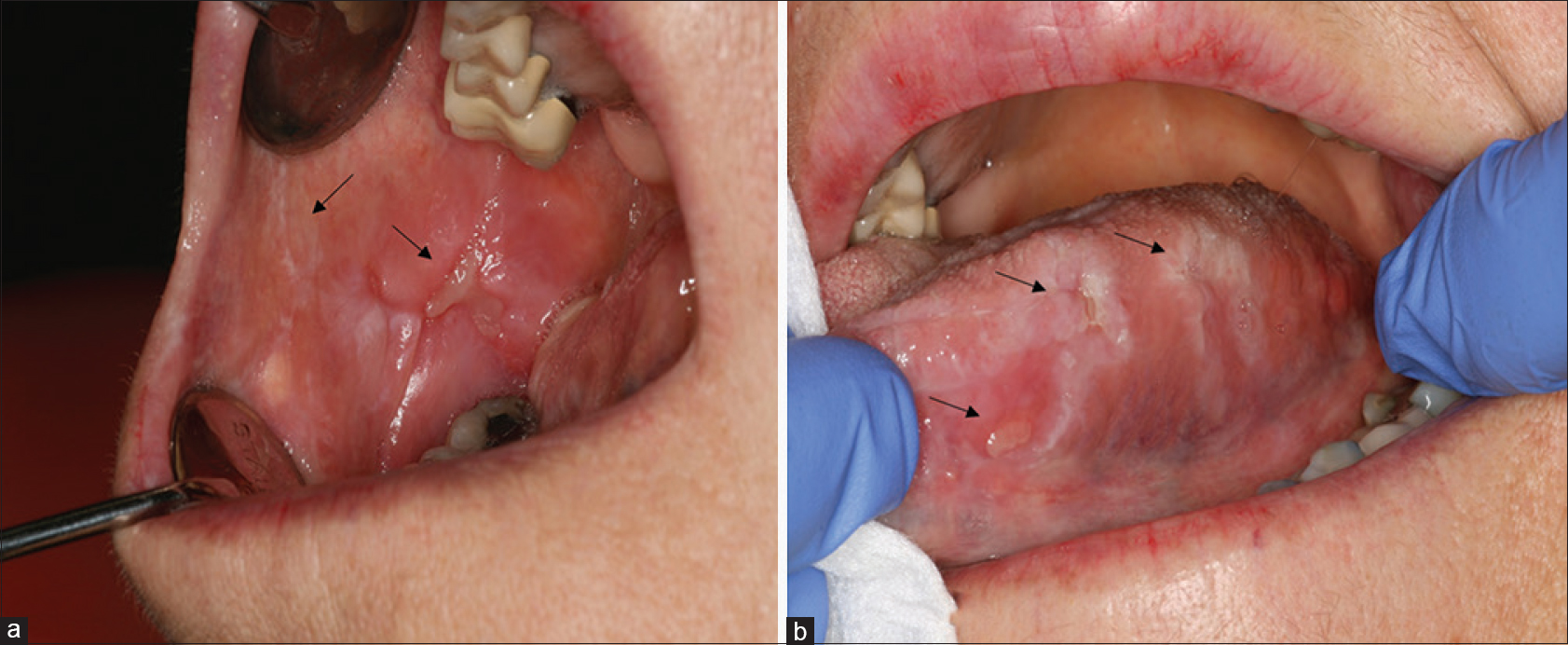 |
| Figure 1: Buccal mucosa (a) and lateral aspect of the tongue (b) with visible reticular white streaks (arrow) and ulcerative lesions (arrow) |
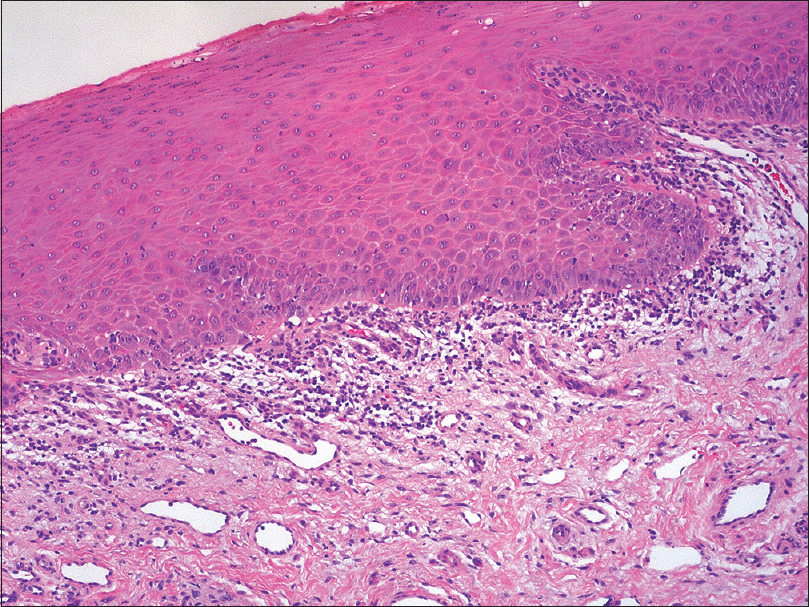 |
| Figure 2: Superficial band-like infiltrate with interface dermatitis (H and E, ×100) |
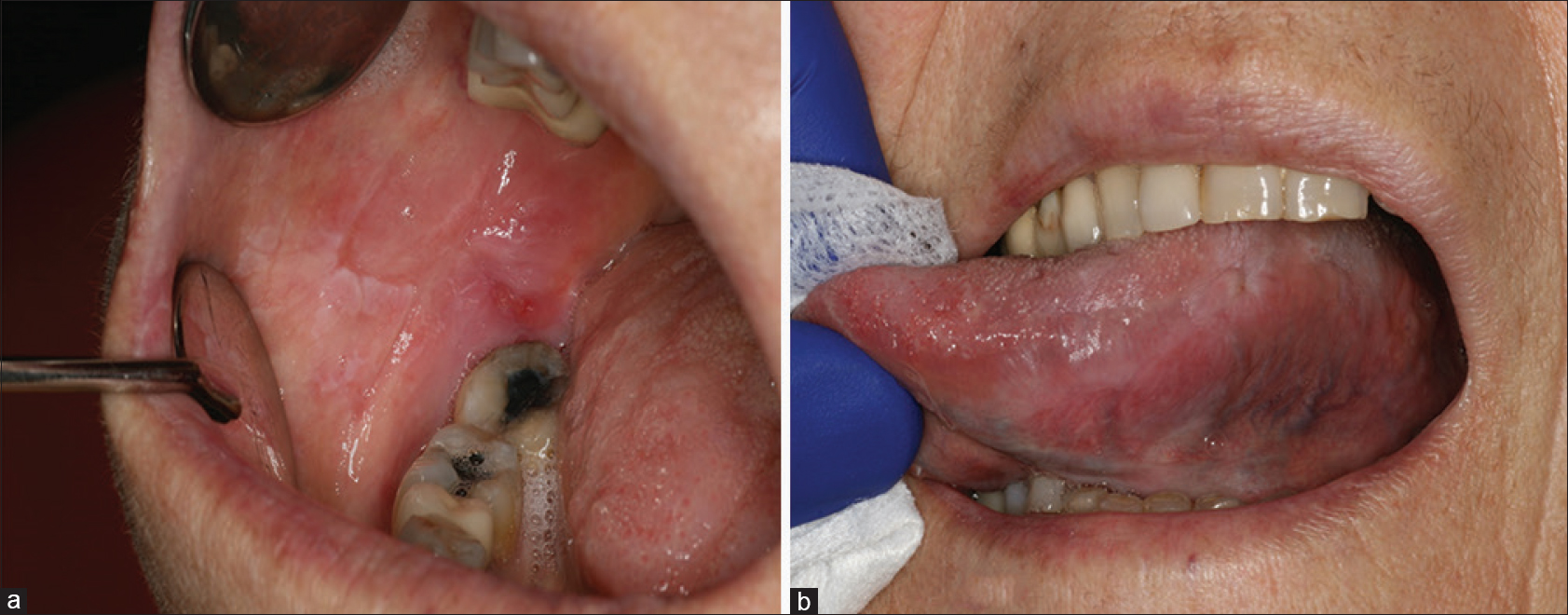 |
| Figure 3: Significant improvement of oral lichenoid lesions after 1-month therapy with topical clobetasol (a and b) |
Oral adverse events induced by new targeted anticancer therapies remain poorly characterized and are frequently described using nonspecific terminology (e.g. “stomatitis” or “mucositis”). However, targeted-therapy-related oral toxicities often display very characteristic changes that differ significantly from mucositis induced by chemotherapeutic agents [Table - 1]. This mainly includes mechanistic target of rapamycin kinase (mTOR) inhibitor-associated stomatitis (mIAS), stomatitis, and benign migratory glossitis associated with multitargeted kinase inhibitors of the vascular endothelial growth factor (VEGF) and platelet-derived growth factor (PDGF) receptors, oral aphthous-like lesions induced by epidermal growth factor receptor (EGFR) inhibitors, hyperpigmentation of the palate with imatinib, ibrutinib-related stomatitis, or hyperkeratotic lesions with B-rapidly accelerated fibrosarcoma (BRAF) inhibitors in monotherapy.[3] Moreover, oral lichenoid reactions have also been observed with a range of targeted therapies, notably with imatinib and to a lesser degree with rituximab. The overall incidence of such oral lichenoid lesions appears even higher with the newly developed immune checkpoint inhibitors. In this context, oral lichenoid reactions may occur alone or in association with cutaneous or nail involvement, as we have recently described with anti-programmed cell death 1 (PD-1) or anti-programmed cell death-ligand 1 (anti-PD-L1) therapies.[4]
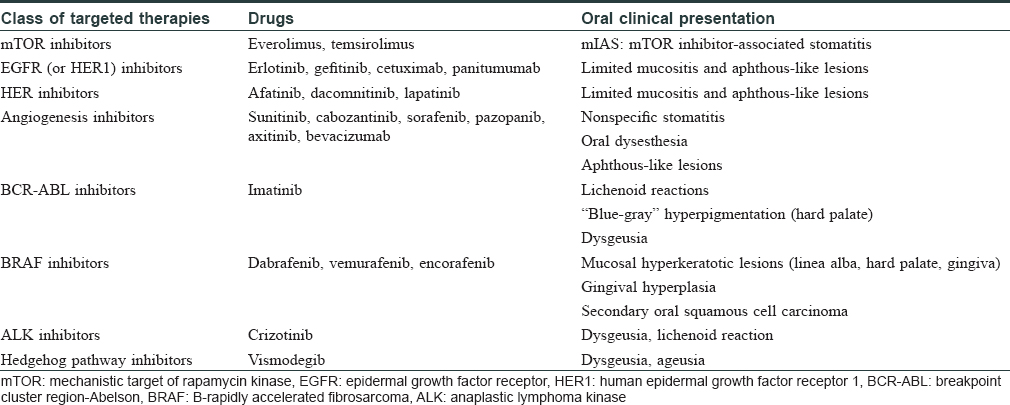
The only emergent oral mucosal toxicity associated with crizotinib is dysgeusia, which affects approximately 20% of patients treated with this drug. 3 Nonspecific stomatitis has been only reported sporadically in a subset of patients treated with this drug. Our case, as well as that recently reported by Ho and Chen, shows that a subset of eruptions induced by crizotinib may be due to a lichenoid reaction, either with skin or oral mucosal involvement.[2] However, the description of these lichenoid toxicities with crizotinib remains isolated until date and this must be confirmed over time. It may also be hypothesized that esophageal ulcerations, which have been described recently with this drug in several patients, may be related to a lichenoid inflammation.[5] The available histopathological analyses, however, do not support this theory.[5] Finally, although the overall incidence of skin rash seems to be higher, no skin or mucosal lichenoid reactions have been reported so far with the next-generation ALK inhibitors (e.g. alectinib, brigatinib).[1]
In conclusion, practitioners should be aware of oral lichenoid reactions occurring in association with targeted therapies, including the ALK inhibitor crizotinib, which may require specific management with topical or systemic corticosteroids.[3],[4] Moreover, the follow-up of treated patients should include a systematic and regular oral examination. Finally, prospective evaluation of the oral region in patients treated with new-generation ALK inhibitors should also be promoted, to check whether this observation corresponds to a class effect or not.
Acknowledgement
The authors thank Dr. Beatrice Herbault-Barres, MD (Pathology department of Institut Claudius Regaud and Cancer University Institute of Toulouse Oncopole, Toulouse, France) for providing histopathology figures.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient has given her consent for her images and other clinical information to be reported in the journal. The patient understands that name and initials will not be published and due efforts will be made to conceal identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Camidge DR, Kim HR, Ahn MJ, Yang JC, Han JY, Lee JS, et al. Brigatinib versus crizotinib in ALK-positive non-small-cell lung cancer. N Engl J Med 2018;379:2027-39.
[Google Scholar]
|
| 2. |
Ho YH, Chen CL. Crizotinib-induced lichenoid drug eruption in a patient with lung cancer. Cutis 2018;102:403-6.
[Google Scholar]
|
| 3. |
Vigarios E, Epstein JB, Sibaud V. Oral mucosal changes induced by anticancer targeted therapies and immune checkpoint inhibitors. Support Care Cancer 2017;25:1713-39.
[Google Scholar]
|
| 4. |
Sibaud V, Eid C, Belum VR, Combemale P, Barres B, Lamant L, et al. Oral lichenoid reactions associated with anti-PD-1/PD-L1 therapies: Clinicopathological findings. J Eur Acad Dermatol Venereol 2017;31:e464-9.
[Google Scholar]
|
| 5. |
Sawada T, Maeno K, Joh T. Esophageal ulcer in a lung cancer patient. Crizotinib-induced esophageal injury. Gastroenterology 2015;149:e6-7.
[Google Scholar]
|
Fulltext Views
3,864
PDF downloads
3,167





