Translate this page into:
Cutaneous protothecosis in an immunocompetent host
Correspondence Address:
Varadraj Vasant Pai
Department of Dermatology, Goa Medical College, Bambolim - 403 202, Goa
India
| How to cite this article: Rajan A, Pai VV, Shukla P. Cutaneous protothecosis in an immunocompetent host. Indian J Dermatol Venereol Leprol 2020;86:414-417 |
Sir,
The Prototheca species are achlorophyllic algae that do not possess chloroplast or pyrenoids and behave as saprophytes.[1] They can infect both humans and animals. Human protothecosis is rare, and is predominantly reported in immunocompromised patients.[2] The clinical manifestations of protothecosis can be classified into three forms: cutaneous (66%), olecranon bursitis (15%) and disseminated or systemic infections (19%).[2],[3] The typical presentation commonly occurs on the face and extremities as erythematous plaques, nodules or superficial ulcers.[4] We report a case of cutaneous protothecosis in an immunocompetent host.
A 69-year-old male farmer, presented with a three-month history of an asymptomatic lesion over the right forearm, which was accidentally detected when the patient was admitted for furosemide-induced hypersensitivity syndrome, which was treated symptomatically without systemic corticosteroids. History of contact with soil, plants and trees with bare hands and recurrent trauma secondary to it was noted. There was no history of application of any topical medications. Clinical cutaneous examination revealed a well-defined plaque with an irregular border, measuring 5 × 3 cm, over the extensor aspect of the right forearm. The surface was studded by skin-colored to hyperpigmented papules, few isolated and few coalesced to form a reticulate network with areas of intermittent atrophy and scarring, mimicking atrophoderma vermiculatum. Few satellite lesions were noted [Figure - 1]. The differential diagnosis considered were atrophoderma vermiculatum, keratosis pilaris atrophicans and anetoderma. Haematological and biochemical investigations were within the normal range. Potassium hydroxide preparation from skin scrapings was negative. Fungal culture from the lesional surface did not show any growth. Cartridge-based nucleic acid amplification test for tuberculosis was negative. Enzyme-linked immunosorbent assay for HIV was non-reactive. Dermoscopy of the lesion showed a distorted pigment network, whitish areas suggestive of fibrosis, clusters of yellowish-brown globules and multiple pinkish structureless areas [Figure - 2].
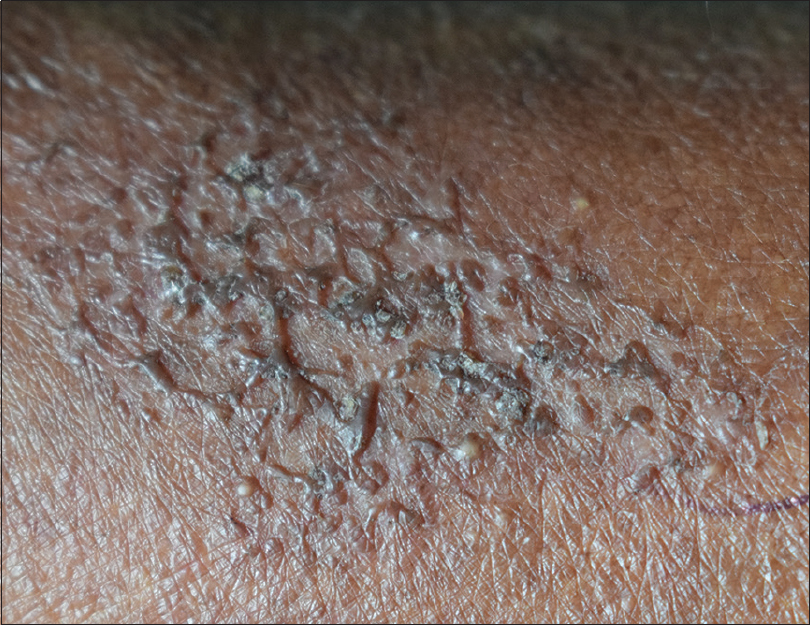 |
| Figure 1: Hyperpigmented papules in a reticulate network, with areas of intermittent atrophy |
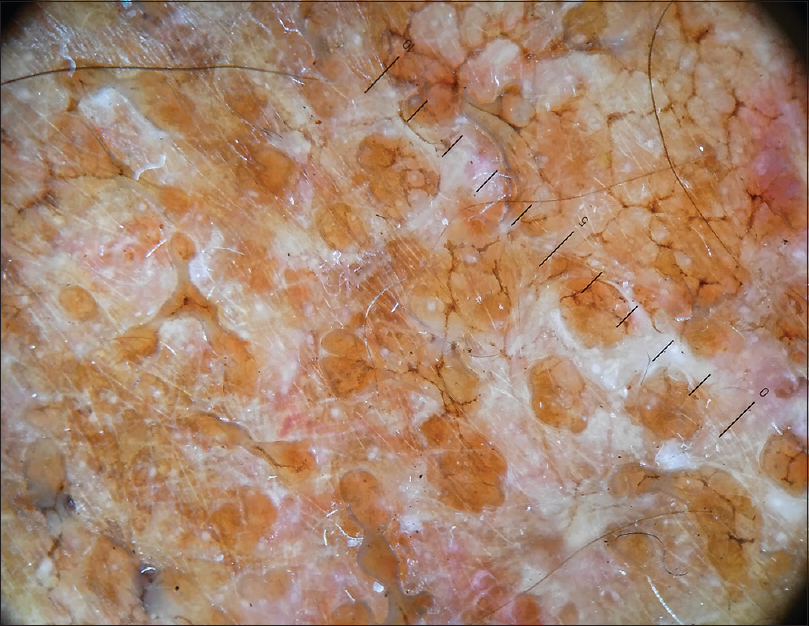 |
| Figure 2: Dermoscopy showing a distorted pigment network, whitish areas suggestive of fibrosis, clusters of yellowish-brown globules and multiple pinkish structureless areas (HEINE DELTA 20T Dermatoscope, polarised light 10×) |
Histopathological examination of the lesion showed that the upper and mid dermis had a band-like diffuse infiltrate of histiocytes with abundant cytoplasm surrounded by focally dense lymphoplasmocytic infiltrate with several eosinophils. On periodic acid Schiff (PAS) staining, numerous PAS positive bodies packed together forming a symmetrical morula or cartwheel-like structure were seen within the histiocytes [Figure - 3] and [Figure - 4]. Therefore, a diagnosis of cutaneous protothecosis was made.
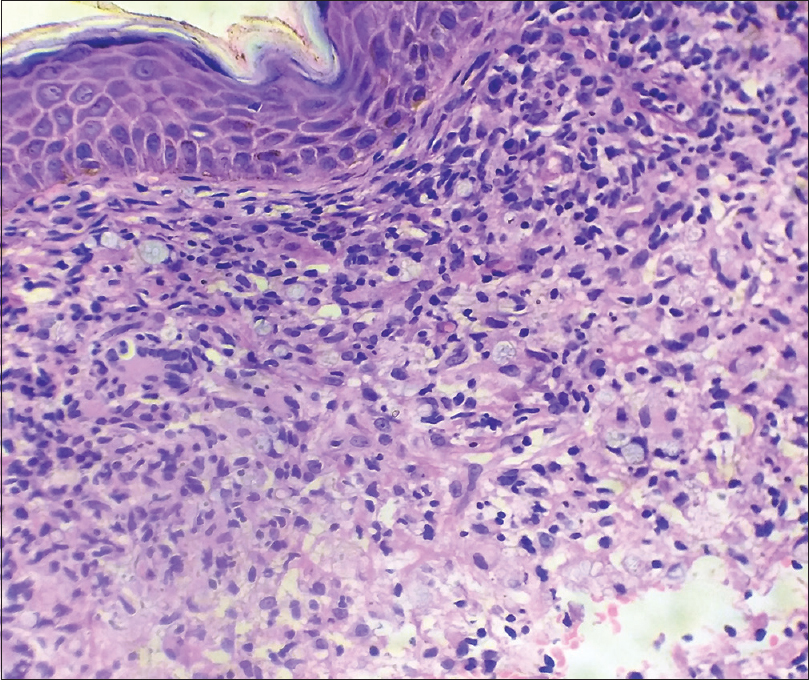 |
| Figure 3: Band like diffuse infiltrate of histiocytes with abundant cytoplasm, with morula-like structures surrounded by focally dense lymphoplasmacytic infiltrate with several eosinophils (H and E,×400) |
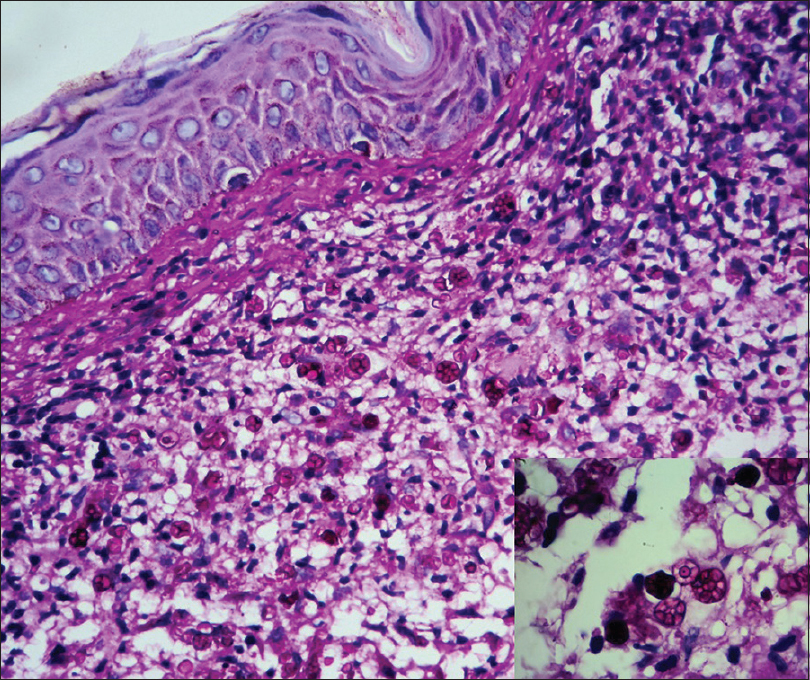 |
| Figure 4: Periodic acid Schiff (PAS) stain with numerous PAS-positive bodies packed together forming morula or cartwheel-like structures, seen within the histiocytes (PAS, ×400). Inset – symmetrical morula like structures as seen in Prototheca wickerhamii (histopathology picture ×1000) |
The patient was treated with tablet itraconazole 200 mg twice a day for 3 months. He showed significant improvement at the end of 3 months and was later lost to follow up [Figure - 5] and [Figure - 6].
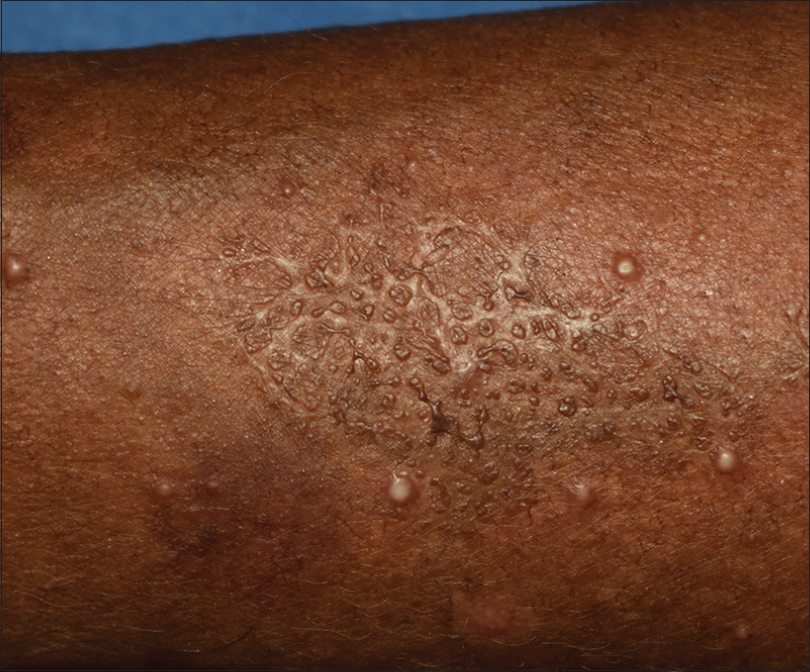 |
| Figure 5: Lesion on the right forearm showing significant improvement with 3 months of treatment |
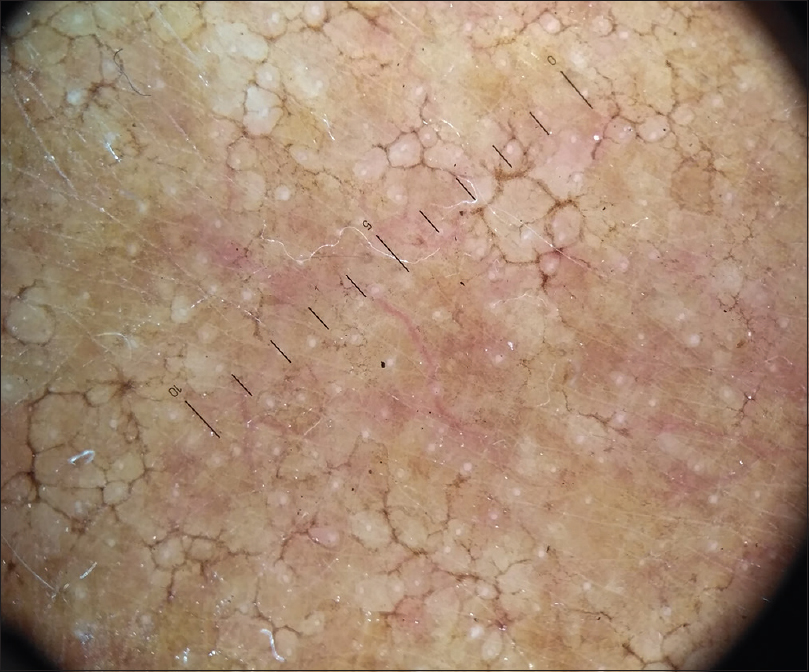 |
| Figure 6: Dermoscopy of the lesion after 3 months treatment with itraconazole, showing reduced yellowish.brown globules |
The Prototheca species is an uncommon cause of human infection in both immunocompetent and immunocompromised patients. Prototheca species was first isolated by Kruger in 1894, while the first description of human infection was made by Davies and colleagues in 1964.[3],[5]
More than 160 cases of protothecosis infecting humans have been reported worldwide, with one case of disseminated protothecosis reported from India.[6],[7]
Prototheca species are spherical unicellular organisms ranging from 3 to 30 μm in diameter. They do not possess glucosamine, a specific fungal cell wall component, or muramic acid, a specific component found in bacterial cell walls.[3] P. wickerhamii and P. zopfii are the species reported to cause infections in humans, with P. wickerhamii being more common of the two.[3] They consist of sporangia with thick, double-layer walls filled with multiple endospores. The sporangia are characteristic of the Prototheca spp and tend to form morula-like structures that have internal septation with a cartwheel-like appearance.[4] The morula forms appear symmetrical in P. wickerhamii, whereas in other species of the Prototheca genus, the morula has a more random internal segmentation without the cartwheel-like appearance.[3],[4] Symmetrical morula-like structures were also seen in our patient.
Prototheca spp. are globally ubiquitous and are found in trees, grass, soil, fresh water and salt water, waste water, animals such as cattle, deer and dogs, animal excrement and food items such as butter, potato peel, cow's milk and bananas.[3] The postulated mode of cutaneous infection is through traumatic inoculation[4]. The incubation period for disease presentation is not well documented and is speculated to range from a week to months.[3]
The organism is of low virulence in patients with an intact immune system, and is common in patients with underlying immunosuppression due to infections like HIV or those on systemic steroids, hematologic or solid tissue malignancy, organ transplant or diabetes mellitus, adrenal insufficiency or occasionally in an immunocompetent patient.[2],[3],[8] A study by Tseng et al. revealed that adrenal insufficiency is a significant risk factor for cutaneous protothecosis.[8] The infection usually spreads indolently in local areas.[3]
Exposed areas including the upper and lower extremities are the most common sites to be affected. 1 The cutaneous lesions can be in the form of erythematous plaques, pustules, papules, nodules, verrucous lesions, pyodermic and herpetiform lesions, vesicles, ulcers and hypopigmented or atrophic lesions.[3] The presentation in immunocompetent patients are often localized papules and pustules. Lesions may be present for months to years, changing gradually in size and shape.[4],[9] The condition may be underdiagnosed as clinical findings are subtle and may be mistaken for scarring in the elderly population. Even in this case, the diagnosis was incidental. The lesions in our case mimicked atrophoderma vermiculatum.
Among the differential diagnoses which were considered, atrophoderma vermiculatum and keratosis pilaris atrophicans are commonly seen since childhood and predominantly localized to the face; while anetoderma, in addition to atrophy, is characterized by loss of normal skin elasticity. One close differential diagnosis would be cutaneous coccidioidomycosis; analysis of sporangia can exclude this diagnosis.
Cutaneous biopsies demonstrate a dermal, granulomatous, inflammatory infiltrate often admixed with lymphocytes, neutrophils and eosinophils. Multinucleated giant cells and plasma cells are usually present. On PAS staining in combination with diastase, nonbudding spherical organisms with multiple sporangia containing endospores in a morula- or cartwheel-like appearance are seen, which is the characteristic feature of the Prototheca species.[1],[3]
The confirmative diagnosis of the species can be made by culture on Sabouraud's dextrose agar, a carbohydrate assimilation test, a yeast biochemical card, or an immunofluorescence study using species-specific antibodies and a molecular study assessing small subunit ribosomal DNA.[1]
The standard treatment regimen for protothecosis is not established. In vitro susceptibility tests have demonstrated sensitivity to amphotericin B, itraconazole, fluconazole, voriconazole and also to antibacterials like tetracycline, gentamycin and amikacin.[3] Itraconazole and amphotericin B are the most commonly used drugs, considering the presence of immunodeficiency in many cases. Other antifungals and surgical excision are the other treatment modalities available.[3],[7],[8] since there are only a few reported cases we feel that in addition to the common occurrence in immunocompromised individuals, it can also occur in immunocompetent individuals all test routinely necessary to rule out immunocompromised status in the form of HIV, VDRL, Biochemical profile including diabetes was done.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient has given his consent for his images and other clinical information to be reported in the journal. The patient understands that name and initials will not be published and due efforts will be made to conceal the identity, but anonymity cannot be guaranteed.
Acknowledgement
The authors would like to thank Dr Rajiv Joshi for the histopathological analysis and pictures.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Seok JY, Lee Y, Lee H, Yi SY, Oh HE, Song JS. Human cutaneous protothecosis: Report of a case and literature review. Korean J Pathol 2013;47:575-8.
[Google Scholar]
|
| 2. |
Lu S, Xi L, Qin W, Luo Y, Lu C, Li X. Cutaneous protothecosis: Two new cases in China and literature review. Int J Dermatol 2012;51:328-31.
[Google Scholar]
|
| 3. |
Lass-Flörl C, Mayr A. Human protothecosis. Clin Microbiol Rev 2007;20:230-42.
[Google Scholar]
|
| 4. |
Hillesheim PB, Bahrami S. Cutaneous protothecosis. Arch Pathol Lab Med 2011;135:941-4.
[Google Scholar]
|
| 5. |
Davies RR, Spencer H, Wakelin PO. A case of human protothecosis. Trans R Soc Trop Med Hyg 1964;58:448-51.
[Google Scholar]
|
| 6. |
Todd JR, King JW, Oberle A, Matsumoto T, Odaka Y, Fowler M, et al. Protothecosis: Report of a case with 20-year follow-up, and review of previously published cases. Med Mycol 2012;50:673-89.9
[Google Scholar]
|
| 7. |
Mathew LG, Pulimood S, Thomas M, Acharya MA, Raj PM, Mathews MS. Disseminated protothecosis. Indian J Pediatr 2010;77:198-9.
[Google Scholar]
|
| 8. |
Tseng HC, Chen CB, Ho JC, Cheng YW, Huang HW, Sun PL, et al. Clinicopathological features and course of cutaneous protothecosis. J Eur Acad Dermatol Venereol 2018;32:1575-83.
[Google Scholar]
|
| 9. |
Woo JY, Suhng EA, Byun JY, Choi HY, Sung SH, Choi YW. A Case of Cutaneous Protothecosis in an Immunocompetent Patient. Ann Dermatol 2016;28:273-274. doi:10.5021/ad.2016.28.2.273.
[Google Scholar]
|
Fulltext Views
6,739
PDF downloads
2,422





