Translate this page into:
Cutaneous squamous cell carcinoma in a patient with Lynch syndrome
2 Department of Dermatology, College of Medicine, Qassim University, Qassim, Saudi Arabia
Correspondence Address:
Sarah Alsukait
Department of Dermatology, College of Medicine, King Saud University, P. O. Box 7805, Riyadh 11472
Saudi Arabia
| How to cite this article: Alsukait S, Almohsen Z, Alqarawi S, Alsaif F. Cutaneous squamous cell carcinoma in a patient with Lynch syndrome. Indian J Dermatol Venereol Leprol 2020;86:407-409 |
Sir,
Lynch syndrome or hereditary nonpolyposis colorectal cancer is the most common form of hereditary colorectal cancer. It is characterized by positive family history, early-onset and the development of metachronous cancers such as endometrial, ovarian, stomach, small bowel, pancreatic, hepatobiliary, brain and urinary tract cancers. It is inherited as an autosomal dominant syndrome and characterized by a germline mutation in mismatch repair genes. Muir–Torre syndrome is a variant of Lynch syndrome, with a higher risk of skin tumors such as sebaceous adenoma, sebaceous carcinoma or keratoacanthoma.[1] Cutaneous and noncutaneous squamous cell carcinomas are very rare in Lynch syndrome.[2] Herein, we report a patient with Lynch syndrome, with a history of colon cancer, who developed cutaneous squamous cell carcinoma.
A 37-year-old woman from Saudi Arabia underwent subtotal colectomy in 2007 and the pathology revealed a T1 (intramucosal with no stalk invasion), N0 and M0 adenocarcinoma of the colon. She did not receive chemotherapy or radiotherapy and has not developed recurrence till date. She had a strong family history of colon cancer, through her mother, sister and brother. Genetic testing for a germline mutation in the adenomatous polyposis coli gene was negative. In 2017, she presented with a lesion on the neck, that had progressively grown over 2 months, associated with intermittent pain, bleeding and ulceration. She had no family history of skin cancers. On examination, we observed a tender exophytic growth, measuring 3 × 4 cm in size and partially ulcerated over the lower left side of the neck with no palpable regional lymph nodes [Figure - 1]. Punch biopsies were obtained from the lesion which showed keratinizing squamous epithelium with focal atypia. Computed tomography of the head and neck revealed a soft-tissue, cutaneous mass in the left side of the lower neck with no underlying extension and multiple cervical lymph nodes. Computed tomography showed no radiologic evidence of metastasis. The tumor was then managed with wide local excision and was identified as keratinizing squamous cell carcinomas, that was moderately differentiated [Figure - 2]. Immunohistochemical studies showed positive staining for MutS Homolog 2 (MSH-2), MutL Homolog 1 (MLH-1) and Postmeiotic Segregation Increased 2 (PMS-2) and focally weak positivity for MutS Homolog 6 (MSH-6) [Figure - 3]. Immunohistochemical staining of the patient's colonic adenocarcinoma showed a lack of expression of MSH-6 [Figure - 4].
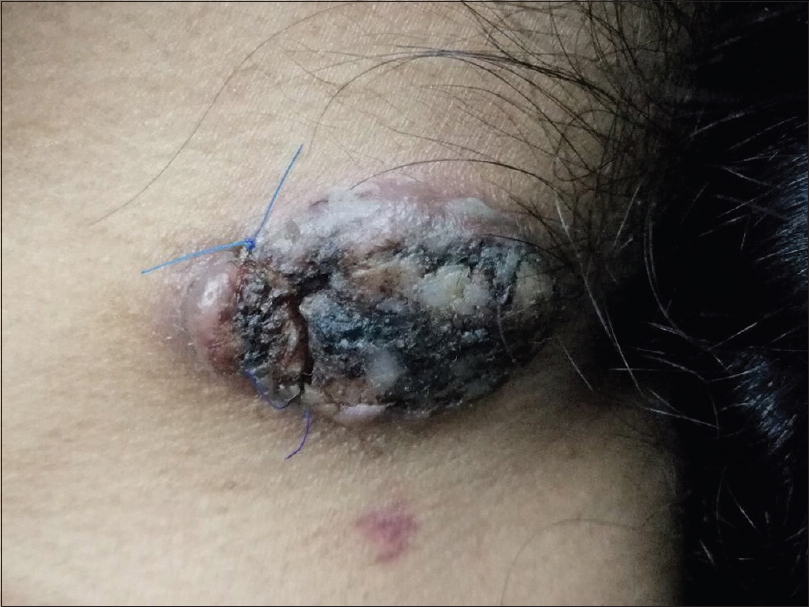 |
| Figure 1: Squamous cell carcinoma over the left lower neck |
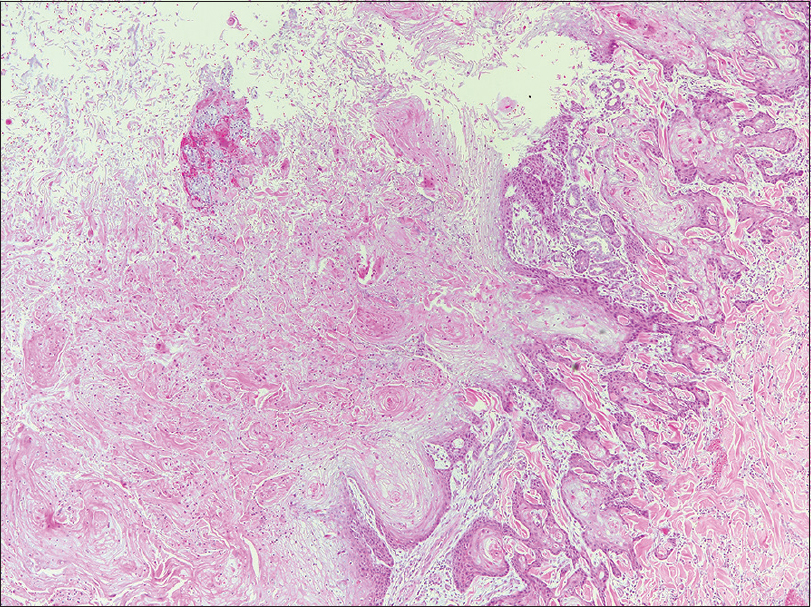 |
| Figure 2: Moderately differentiated keratinizing squamous cell carcinoma (H and E stain, original magnification, ×100 |
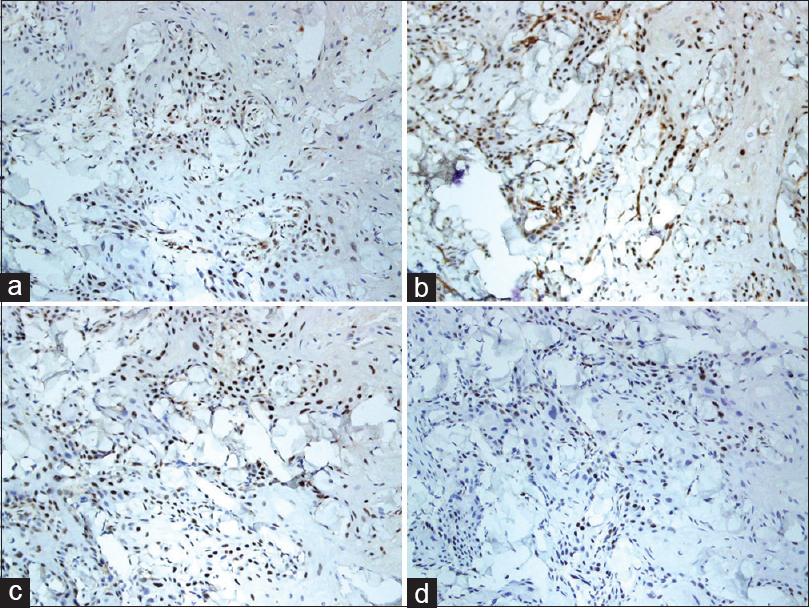 |
| Figure 3: Immunohistochemical staining of the patient's squamous cell carcinoma showing strong positivity of (a) MSH-2 (×200), (b) MLH-1 (×200), (c) PMS-2 (×200) and (d) weak focal positivity of MSH-6 (×200) |
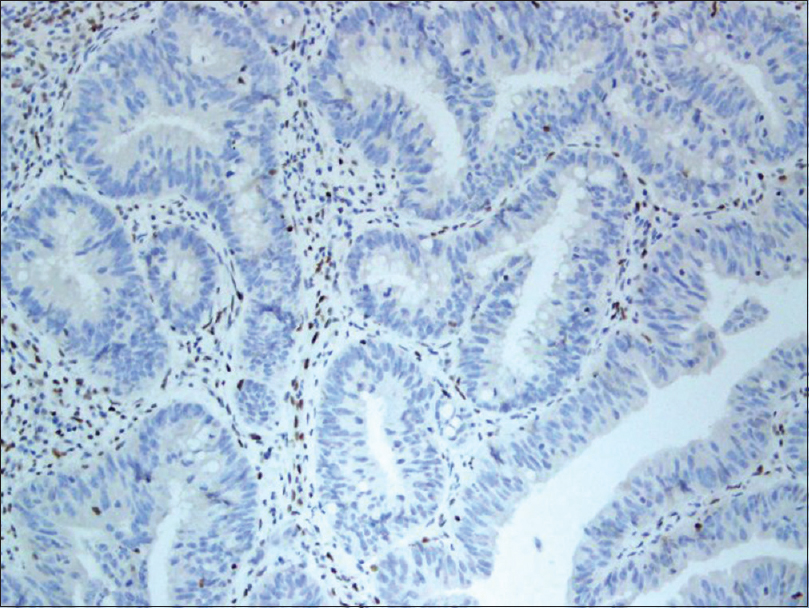 |
| Figure 4: Immunohistochemical staining of the patient's colonic adenocarcinoma showing lack of expression of MSH-6 (×200) |
Lynch syndrome (previously referred to as hereditary nonpolyposis colorectal cancer) is associated with a predisposition to various malignancies at a young age. It is characterized by a germline mutation in one of the mismatch repair genes (most commonly MLH-1, MSH-2, MSH-6 or PMS-2).[1] Germline mutations in MSH-6 account for 18% of Lynch syndrome cases and result in tumors lacking MSH-6 expression, as observed in our patient's colon cancer.[3] The Muir–Torre variant is characterized by the presence of sebaceous adenomas, sebaceous carcinomas and/or keratoacanthomas with visceral malignancies.[1]
In a study conducted in the Dutch population, malignant skin tumors were found in 8.2% of Lynch syndrome patients. An important finding of this study was the twelve-fold increased risk, at the age of 60 years; of developing a squamous cell carcinomas or sebaceous carcinoma in their population of patients with Lynch syndrome, compared to the general Dutch population.[4] Squamous cell carcinomas is not considered part of the Lynch syndrome-associated cancer spectrum. However, it was described in a few case reports. In 2014, Amjad et al. reported the first case of squamous cell carcinomas in the duodenum, showing loss of MSH-2 and MSH-6 expression, in a patient with Lynch syndrome, with a history of colorectal adenocarcinoma and MSH-2 gene deletion.[2] In 2015, Sorscher described a case of a molecularly confirmed germline mutation in MLH-1, along with colon cancer and squamous cell carcinomas, both showing lack of expression of MLH-1, in a 54-year-old man.[5] Our patient had a history of colon cancer which showed a lack of expression of MSH-6. However, her skin squamous cell carcinoma showed strong, positive expression of MSH-2, MLH-1 and PMS-2 and weak focal positivity of MSH-6.
This is a new case of a tumor that is not part of the Lynch syndrome spectrum developing in a patient with Lynch syndrome. Squamous cell carcinomas is relatively common, making it difficult to establish a true link between this tumor and germline mutations. It may be best in accordance with the reported cases, to consider the increased risk and perform annual skin examinations to identify any suspicious skin lesions.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patients have given their consent for their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Lynch HT, Lynch PM, Lanspa SJ, Snyder CL, Lynch JF, Boland CR. Review of the Lynch syndrome: History, molecular genetics, screening, differential diagnosis, and medicolegal ramifications. Clin Genet 2009;76:1-8.
[Google Scholar]
|
| 2. |
Amjad AI, Singhi AD, Balaban EP, Dudley B, Brand RE, Bahary N. First reported case of a squamous cell carcinoma arising in the duodenum in a patient with Lynch syndrome. Int J Clin Exp Pathol 2014;7:8988-95.
[Google Scholar]
|
| 3. |
Houlleberghs H, Goverde A, Lusseveld J, Dekker M, Bruno MJ, Menko FH, et al. Suspected lynch syndrome associated MSH6 variants: A functional assay to determine their pathogenicity. PLoS Genet 2017;13:e1006765.
[Google Scholar]
|
| 4. |
Adan F, Crijns MB, Zandstra WSE, Bekkenk MW, Bleeker FE, Dekker E, et al. Cumulative risk of skin tumours in patients with lynch syndrome. Br J Dermatol 2018;179:522-3.
[Google Scholar]
|
| 5. |
Sorscher S. A case of squamous cell carcinoma of the skin due to the molecularly confirmed lynch syndrome. Hered Cancer Clin Pract 2015;13:12.
[Google Scholar]
|
Fulltext Views
4,223
PDF downloads
2,098





