Translate this page into:
Cutaneous ulcer with thrombogenic vasculopathy in a patient receiving bevacizumab
Corresponding author: Dr. Aina Vila-Payeras, Department of Dermatology, Son Llàtzer Hospital, P alma de Mallorca, Spain. ainavila91@hotmail.com
-
Received: ,
Accepted: ,
How to cite this article: Vila-Payeras A, Iglesias-González M, Terrasa-Sagristá F, Parera-Amer E. Cutaneous ulcer with thrombogenic vasculopathy in a patient receiving bevacizumab. Indian J Dermatol Venereol Leprol 2021;87:268-70.
Sir,
Bevacizumab is a recombinant humanized anti-VEGF (vascular endothelial growth factor) monoclonal antibody that inhibits angiogenic and vasculogenic processes. Currently, it is widely used in the treatment of different metastatic neoplasms, including ovarian cancer.1 Although its toxicity is lower than other similar drugs, reports of impaired wound healing have been described, as in the patient we present below.
We report the case of a 70-year-old woman with a history of ovarian adenocarcinoma diagnosed in 2016. She had been treated with bevacizumab (dose 7.5 mg/kg every 3 weeks) for pulmonary, peritoneal and hepatic tumor progression since august 2017. In November 2018, after an incidental trauma, she developed a pretibial ulcer on the left leg. The ulcer was very painful and refractory to standard topical treatments. Two months later, bevacizumab was stopped due to cataract surgery with an unexpected concomitant improvement of the ulcer. The patient was rechallenged with bevacizumab and the wound gradually worsened. Physical examination revealed an ulcerated lesion of 2 centimeters on the pretibial left leg. The ulcer had raised borders of erythematous color while the floor was covered by fibrinous tissue [Figure 1a]. The patient reported an intense burning sensation and pain which was exacerbated by periods of standing. A punch biopsy of the borders showed a predominantly septal dermato-panniculitis with signs of capillary thrombotic vasculopathy with no signs of vasculitis nor leukocytoclasia [Figure 2a and b]. Tissue cultures were positive for methicillin-resistant staphylococcus aureus and negative for fungal or mycobacterial infections. A basic metabolic panel, complete blood count, autoimmunity and thrombophilia screening test (including lupus anticoagulant, anti-cardiolipin antibody, anti-β2 glycoprotein 1 antibody, activated protein C resistance, fibrinogen tests, factor V Leiden, prothrombin mutation and basal homocysteine levels) showed no alterations. The rest of complementary studies were normal, excluding other differential diagnosis such as livedoid vasculopathy, embolisms or vasculitis.
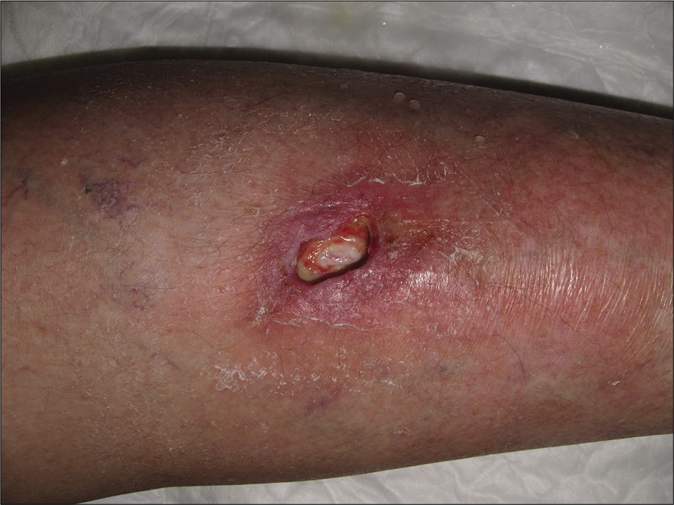
- Clinical course. Cutaneous skin ulcer at first visit
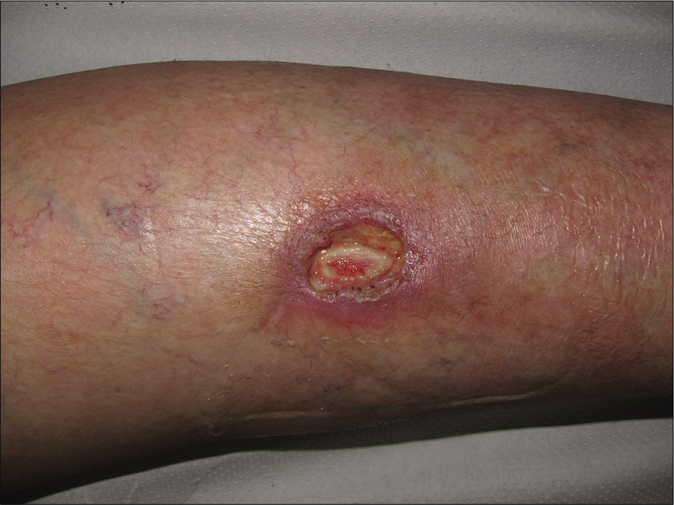
- Clinical course. Granulation tissue developing progressively after 4 weeks of bevacizumab stop
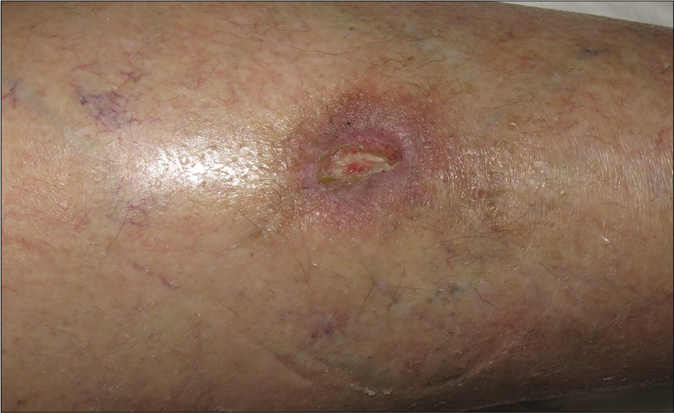
- Clinical course. Granulation tissue developing progressively after 8 weeks of bevacizumab stop
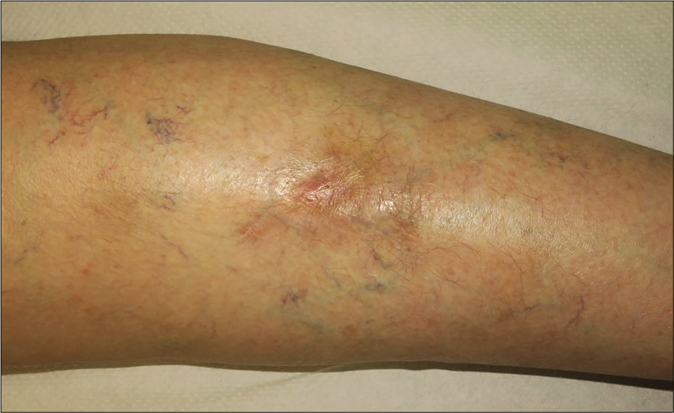
- Clinical course. Complete healing of the wound 3 months after bevacizumab discontinuation
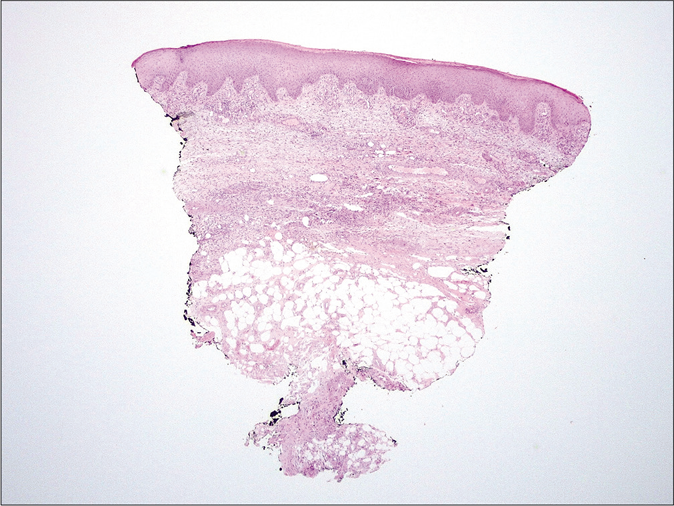
- Histology. Predominantly septal dermato-panniculitis with associated signs of capillary thrombotic vasculopathy with no evidence of vasculitis (H and E, ×10)
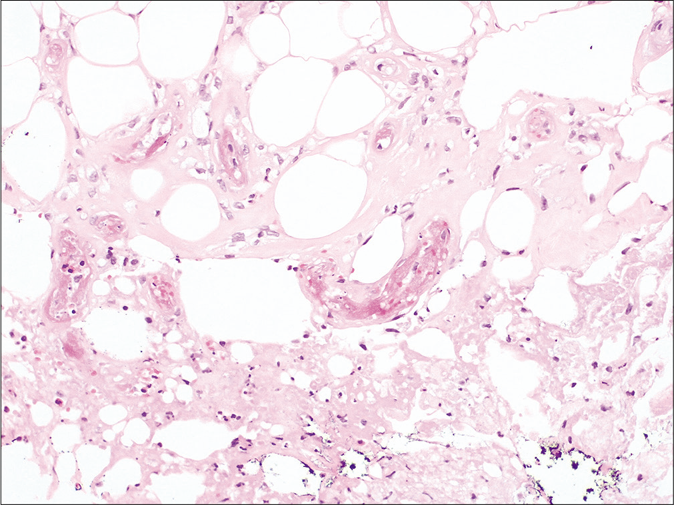
- Histology. Fibrin thrombus inside the capillary vessels of the hypodermis without any signs of vasculitis nor inflammation (H and E, ×200)
Giving these findings, the diagnosis of cutaneous ulcer due to thrombotic vasculopathy in the context of bevacizumab treatment was established. Initial treatment with Trimethoprim-sulfamethoxazole and local silicone dressings was started. Bevacizumab therapy was discontinued permanently and after 4 [Figure 1b] and 8 weeks [Figure 1c] notably improvement was observed with progressive approximation of the ulcers borders with concomitant growing of granulation tissue. After three months, the wound was practically epithelialized without recurrences [Figure 1d].
Bevacizumab is a VEGF inhibitor available since 2005 as an antineoplastic agent, specially used in the treatment of metastatic colorectal carcinoma, non-squamous non-small cell lung cancer, HER2-negative breast and ovarian cancer. Other well-known VEGF inhibitors include sorafenib, sunitinib and axitinib. Mucocutaneous adverse effects of these agents include the hand-foot skin reaction, keratoacanthoma, squamous cell carcinoma, hair depigmentation, stomatitis and thrombogenic vasculopathy.1
The effectiveness of Bevacizumabs’ anti-tumor action has been demonstrated but its mechanism of action is not certainly understood. It has a fundamental role in vasculogenesis and angiogenesis, leading to a decrease in the number of endothelial cells and microcapillaries in the tumor tissue, as well as a decrease in vascular permeability.2
As Kiuru et al.1 first reported a case of cutaneous thrombogenic vasculopathy associated with Bevacizumab and because angiogenesis is involved in physiological processes such as wound healing, studies have shown that Bevazicumab could interfere with them and increase the risk of delayed wound healing. Inhibition of VEGF would lead to increased vascular tone and so dysregulated vasoconstriction in the skin may disrupt normal tissue repair.
Peters et al.3 reported a case of ulceration necrosis of striae distensae produced by corticosteroids in patients with brain tumors on treatment with bevacizumab. Animal models had shown that the inhibition of the wound healing process at the level of the dermis can be dose-dependent and could be reversible when interrupting the administration of the drug.4
In our patient, as in the case of Murayama et al.,5 we suspected that the impaired wound healing could be due to the neutralization of VEGF by bevacizumab. This was supported by the fact that improvement of the ulcer was observed when the treatment was stopped and worsened after the reintroduction of bevacizumab. After permanent withdrawal of bevacizumab lesions completely resolved.
In conclusion, we present the case of a 70-year-old woman treated with Bevacizumab for a metastatic ovarian cancer, who developed a refractory painful skin ulcer that healed completely after stopping this treatment. Dermatologists should be aware of the thrombotic events in the skin with antiangiogenic therapy. because they represent a significant adverse effect associated with their use.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Cutaneous thrombogenic vasculopathy associated with bevacizumab therapy. Dermatol Online J. 2014;20:13030.
- [Google Scholar]
- Cutaneous vasculopathy as an adverse effect of the anti-vascular endothelial growth factor agent axitinib. JAMA Dermatol. 2016;152:222-3.
- [CrossRef] [PubMed] [Google Scholar]
- Ulceration of striae distensae in high-grade glioma patients on concurrent systemic corticosteroid and bevacizumab therapy. J Neurooncol. 2011;101:155-9.
- [CrossRef] [PubMed] [Google Scholar]
- Impaired wound healing and expansion of a large ulcer after bevacizumab with paclitaxel for skin metastases from breast cancer: Report of a case. Surg Today. 2015;45:498-502.
- [CrossRef] [PubMed] [Google Scholar]
- Is bevacizumab a culprit of intractable skin ulcers? J Dermatol. 2016;43:972-4.
- [CrossRef] [PubMed] [Google Scholar]





