Translate this page into:
Demodex folliculorum associated Bacillus pumilus in lesional areas in rosacea
2 Department of Dermatology, Dermatology Outpatient Polyclinic, University Hospital “Saint-Louis,” Paris, France
3 Department of Bacteriology, Carol Davila University and Central Reference Laboratory Synevo, Bucharest, Romania
Correspondence Address:
Alin Laurentiu Tatu
AL.I.Cuza Street No. 39, Galati, 800101
Romania
| How to cite this article: Tatu AL, Ionescu MA, Cristea VC. Demodex folliculorum associated Bacillus pumilus in lesional areas in rosacea. Indian J Dermatol Venereol Leprol 2017;83:610-611 |
Sir,
Demodex species and Bacillus oleronius play an important role in the inflammatory mechanisms of rosacea. More recently, it has been shown that the cutaneous microbiome plays an important role in several chronic inflammatory skin diseases.[1] Dermoscopic and microbiologic assessment help to identify specific elements for the differential diagnosis and can have a predictive value for severe forms and for the choice of treatment. In this case report, our purpose was to identify possible correlations between clinical features, dermoscopy, Demodex folliculorum and microbial presence in an adult patient with rosacea. We report a case of a patient with erythematotelangiectatic rosacea whose clinical features were particularly inflammatory, with intense red, symmetrical areas on the face accompanied by slight pruritus, burning and stinging.
Clinical examination showed symmetric red areas on the cheeks with filiform spicules, whitish-yellow follicular plugs and telangiectases [Figure - 1].[2] The patient had had no preceding topical or systemic treatment. We made parasitologic and bacteriologic assessments. Parasitologic assessment was guided by dermoscopy and was made by scraping. Dermoscopic pictures were taken using Dermlite ® 3Gen (LLC, Dana Point, CA, USA) at ×10 magnification attached to a digital camera Nikon Coolpix ® P5100. Dermoscopy revealed filiform threads, semi-round white plugs in follicular openings and polygonal vessels associated with linear vessels [Figure - 2]. D. folliculorum was isolated [Figure - 3].
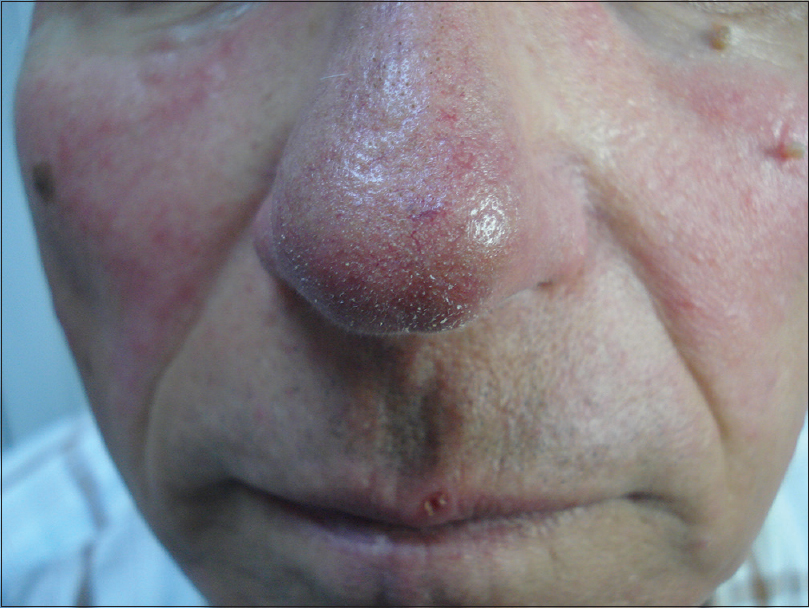 |
| Figure 1: Rosacea – the nasal area |
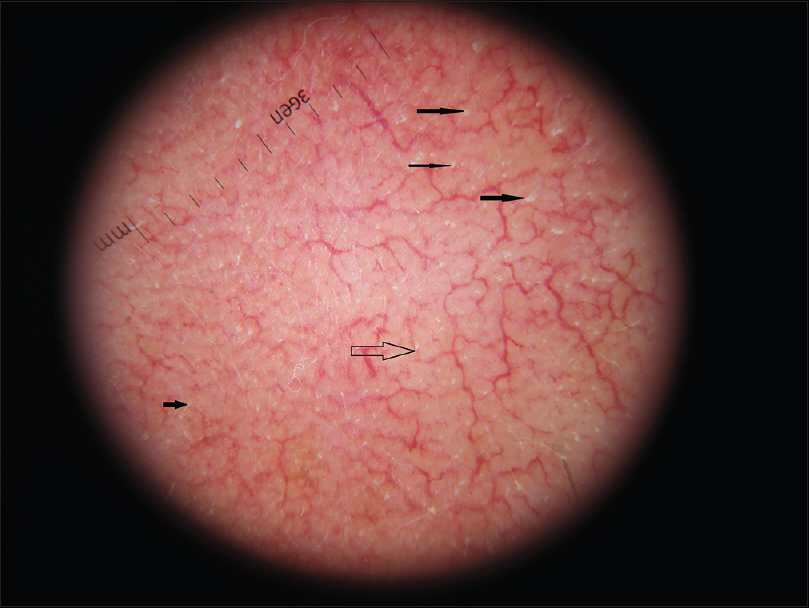 |
| Figure 2: Dermoscopy-Demodex tails, semi-round white-yellowish plugs, polygonal vessels (×10) |
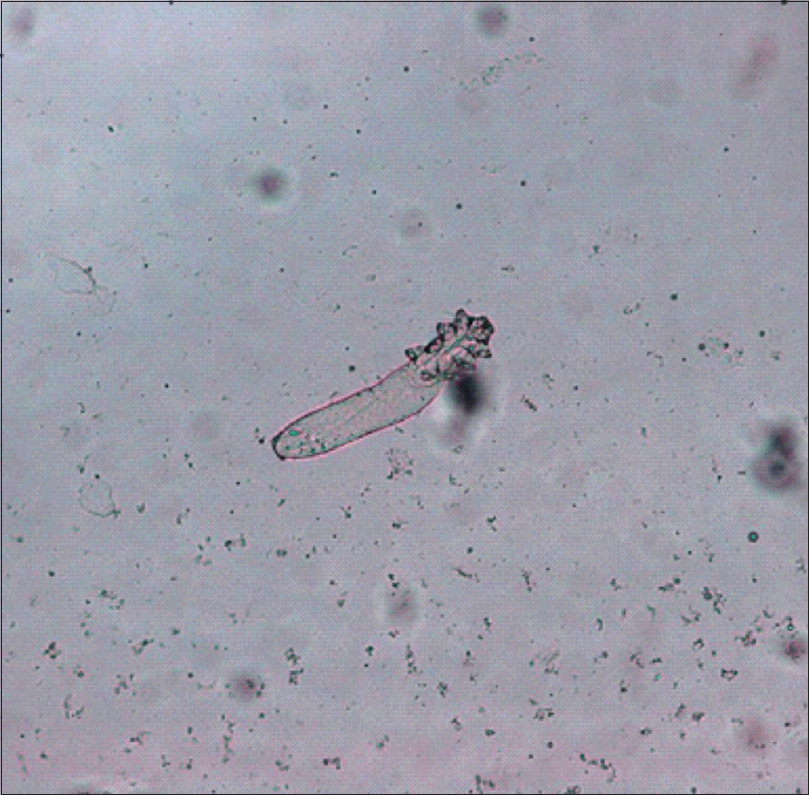 |
| Figure 3: Demodex folliculorum after scraping (optical microscopy, ×20) |
Samples were collected by scraping from the affected area of the skin and from non-lesional skin and by tape stripping using a piece of 2 cm transparent Scotch ® tape applied on both affected and non-affected areas. Scotch ® tape preparation and skin scraping specimen were examined microscopically with objectives ×10 and ×20, assessing D. folliculorum parasitic load. Scraping isolated D. folliculorum only from the lesional skin. D. folliculorum was not found from scraping of non-lesional skin; tapes were negative of D. folliculorum on lesional and non-lesional skin. D. folliculorum isolated by scraping from lesional skin, the scraping specimen of non-lesional skin (without D. folliculorum) and direct specimen (without scraping) from the lesional and nonlesional skin were plated onto culture media. For bacterial cultures, sterile swabs were used and the pad was placed in liquid transport medium (Amies Transport Medium [Copan ®]). Bacteriology samples were spun and then plated on trypticase soy agar and Columbia ® agar with incubation at 37úC for 24 h [Figure - 4]. Direct cultures from lesional and nonlesional skin and cultures from the scraping specimen of nonlesional skin were negative. Identification was performed by matrix-assisted laser desorption/ionization time-of-flight using mass spectrometry MS Microflex LT (Daltonik Bruker ® GmbH, Bremen, Germany) using software FlexControl.[3],[4] The spectra were collected and analyzed using Bruker analyzer database Biotyper (database −5627 SPECIES list). Acceptable score for genus and species identification was considered >2. In this patient with rosacea associated with D. folliculorum, we found positive cultures [Figure - 4] and the matrix-assisted laser desorption/ionization time-of-flight positivity for Bacillus pumilus from lesional skin scrapings. D. folliculorum was not found from scraping of nonlesional skin; tapes were negative of Demodex on lesional and nonlesional skin. No Bacillus was identified in direct cultures from the lesional skin and nonlesional skin and cultures from the scraping specimen of nonlesional skin. B. pumilus is commonly isolated from a variety of environmental sources, particularly feces of animals.[5] B. pumilus has cytotoxic properties, hemolytic activity, can produce lecithinase and has a photolytic action on casein.[6] It is possible that these properties could be related to the development of inflammatory rosacea. Peptidoglycans and structural proteins from bacilli can activate innate immunity receptors such as toll-like receptors, known to induce proinflammatory cytokines (such as interleukin-8, neutrophil chemotactic interleukin-1)[7] and consequent expression of antimicrobial peptides such as cathelicidinLL37 that participate in inflammatory process of rosacea.[8]B. oleronius was previously reported to play a significant role in rosacea. In this case of inflammatory rosacea, we did not find B. oleronius, but we isolated B. pumilus. We were unable to find any previous reports about the presence and the role of B. pumilus in rosacea. The cytotoxicity of B. pumilus could explain the inflammatory clinical features in this case. To assess possible correlations between subtypes of rosacea, dermoscopy features and the microbiome, further studies with large study samples are required.
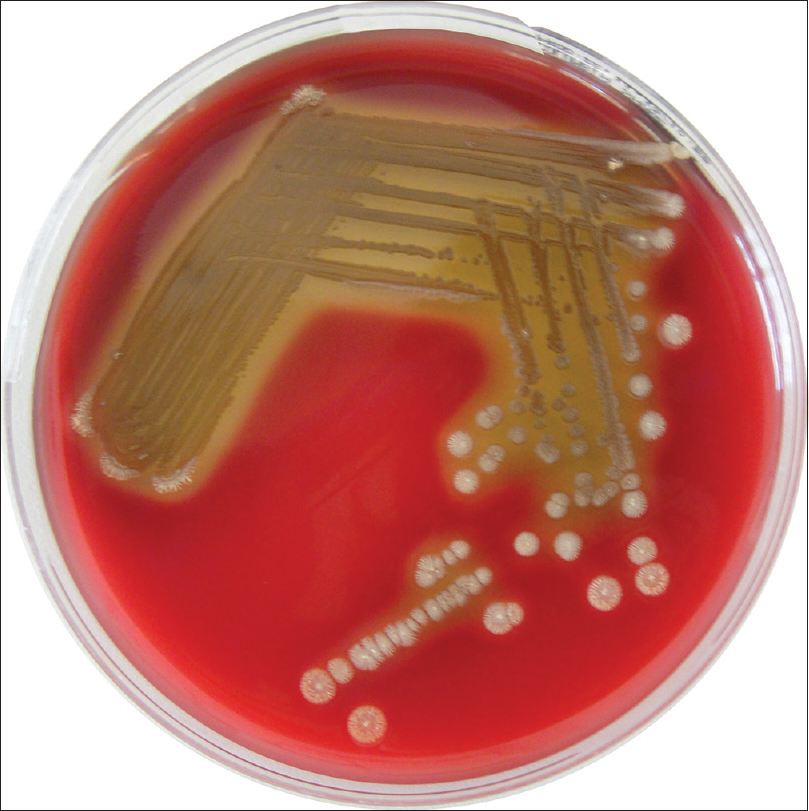 |
| Figure 4: Bacillus pumilus culture at 72 h (rural Colombia - 5% sheep blood) |
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Jarmuda S, O'Reilly N, Zaba R, Jakubowicz O, Szkaradkiewicz A, Kavanagh K. Potential role of Demodex mites and bacteria in the induction of rosacea. J Med Microbiol 2012;61(Pt 11):1504-10.
[Google Scholar]
|
| 2. |
Tatu AL, Clatici V, Cristea V. Isolation of Bacillus simplex strain from Demodex folliculorum and observations about Demodicosis spinulosa. Clin Exp Dermatol 2016;41:818-20.
[Google Scholar]
|
| 3. |
Degand N, Carbonnelle E, Dauphin B, Beretti JL, Le Bourgeois M, Sermet-Gaudelus I, et al. Matrix-assisted laser desorption ionization-time of flight mass spectrometry for identification of nonfermenting Gram-negative bacilli isolated from cystic fibrosis patients. J Clin Microbiol 2008;46:3361-7.
[Google Scholar]
|
| 4. |
Tatu AL, Ionescu MA, Clatici VG, Cristea VC. Bacillus cereus strain isolated from Demodex folliculorum in patients with topical steroid-induced rosaceiform facial dermatitis. An Bras Dermatol 2016;91:676-8.
[Google Scholar]
|
| 5. |
Brophy PF, Knoop FC. Bacillus pumilus in the induction of clindamycin-associated enterocolitis in guinea pigs. Infect Immun 1982;35:289-95.
[Google Scholar]
|
| 6. |
Hoult B, Tuxford AF. Toxin production by Bacillus pumilus. J Clin Pathol 1991;44:455-8.
[Google Scholar]
|
| 7. |
Kang SS, Kauls LS, Gaspari AA. Toll-like receptors: Applications to dermatologic disease. J Am Acad Dermatol 2006;54:951-83.
[Google Scholar]
|
| 8. |
Yamasaki K, Kanada K, Macleod DT, Borkowski AW, Morizane S, Nakatsuji T, et al. TLR2 expression is increased in rosacea and stimulates enhanced serine protease production by keratinocytes. J Invest Dermatol 2011;131:688-97.
[Google Scholar]
|
Fulltext Views
5,926
PDF downloads
1,391





