Translate this page into:
Depigmentation therapies in vitiligo
Correspondence Address:
Devinder Mohan Thappa
Department of Dermatology and STD, JIPMER, Pondicherry - 605006
India
| How to cite this article: Gupta D, Kumari R, Thappa DM. Depigmentation therapies in vitiligo. Indian J Dermatol Venereol Leprol 2012;78:49-58 |
Abstract
Depigmentation therapy in vitiligo is an option in those with extensive vitiligo who have failed to respond to medical therapy and have obvious cosmetic disfigurement due to intervening patchy pigmented areas. Various aspects of this therapy such as the cost, treatment time, course, permanency of depigmentation, side effects, and the possibility of repigmentation should first be discussed with the patient. At present, there is no ideal depigmenting therapy available, but many agents in the market have been in use for many years. Monobenzyl ether of hydroquinone (MBEH) is the mainstay and Food and Drug Administration (FDA) approved in USA but takes many months to depigment and is associated with local side effects and risk of repigmentation. Other agents which are also used are 4-methoxy phenol and 88% phenol. Physical therapies for depigmentation include Q-switched ruby and alexandrite lasers and cryotherapy. Second-line agents which can be explored for depigmentation include imatinib mesylate, imiquimod, and diphencyprone. Many possible experimental agents are being explored like various phenol derivatives, melanoma vaccines, interferon gamma, busulfan, etc. A major lacuna still exists in this area and a lot more research is desirable to give satisfactory cosmesis to these patients with extensive vitiligo.Introduction
Vitiligo, a disease characterized by depigmented macules, is a common cause of cosmetic disfigurement. It occurs worldwide with a prevalence of 0.1 to 2% and has a multifactorial complex pathogenesis. [1]
Vitiligo, in India, is referred to as "ven kushtam," meaning white leprosy. [2] It is associated with profound psychosocial impact on the patient′s quality of life, especially in dark-skinned individuals. [3],[4] In patients with extensive and resistant vitiligo, an attempt to repigment may result in patchy, cosmetically disfiguring pigmentation and hence they prefer to completely depigment their normal pigmented skin. The ultimate goal of depigmentation therapy is to have uniform skin color as patient′s two-toned skin draws more attention.
During depigmentation therapies in vitiligo, cytotoxic potential of chemicals, lasers, cryogen, allergens, vaccines, etc. is used. In this review, we have discussed and compared various established and potential depigmentation agents in existing scientific literature that can be used in extensive and universal vitiligo. We have also tried to throw light on various emerging therapies.
Do we have an Ideal Depigmenting Agent ?
An ideal depigmenting agent should have a potent, rapid, and selective effect on melanocytes, should lead to permanent removal of pigment, and should be nontoxic with minimal side effects. [5] Presently, no agent in market is ideal for depigmentation. [Table - 1] highlights the various depigmenting agents.

Patient Selection
The most important step in depigmentation therapy is to choose an appropriate vitiligo patient. The treating physician should first discuss various aspects of this therapy with the patient, such as the cost, treatment time, course, risk of depigmentation at distant body sites, and stress upon probable permanency of depigmentation, side effects such as contact dermatitis, and the possibility of repigmentation. [6]
It is also very important to make the patient understand the cultural effects depigmentation may have, especially in those with dark skin. Patient must be provided time and assistance in making the decision and family members should be involved in the process too. Depigmentation therapy should be avoided in children less than 12 years of age. [7] As this process is mostly irreversible, younger patients should be given the option of repigmentation lest in few years better technology may become available. [8] [Table - 2] shows the patients in whom depigmenting therapy should be considered [Table - 3] and [Table - 4] shows depigmenting agents.

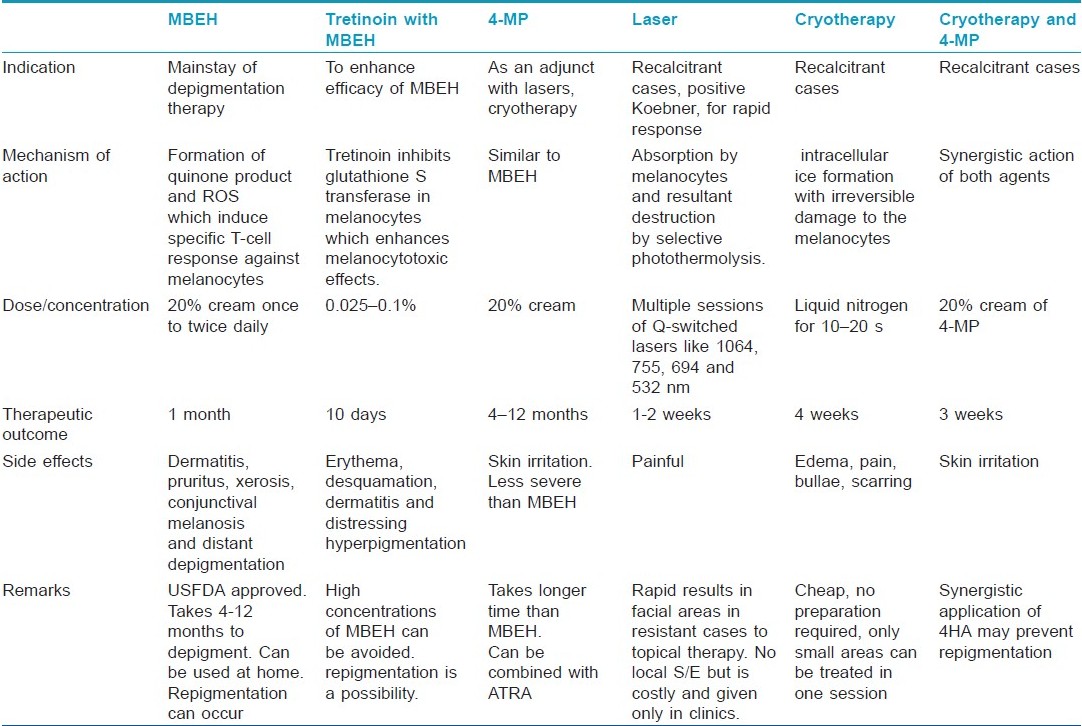
Monobenzyl Ether of Hydroquinone
Monobenzyl ether of hydroquinone (MBEH) is a hydroquinone derivate, also known as monobenzone or by its chemical name, p-(benzyloxy) phenol. At present, it is the most potent depigmenting agent and the mainstay of depigmentation therapy. It is the only drug USFDA approved for depigmentation in vitiligo. [5]
In the late 1930s, the rubber industry began to use a phenol derivative, MBEH, as an antioxidant to reduce the deterioration of rubber. Soon thereafter, they noticed that a group of tannery workers began to develop patches of pigment loss in a distribution that corresponded primarily to areas of the skin that were in contact with these new rubber gloves. [9] This was indistinguishable from vitiligo as it spread to non-exposed sites upon prolonged occupational exposure, suggesting a systemic reaction, though initially it was attributed to sites of contact with the gloves while perspiring. Analysis of the gloves demonstrated the presence of an antioxidant known as agerite alba, or MBEH, and when this compound was removed from the gloves, repigmentation was reported. Though it initially sparked enthusiasm for use of MBEH in all types of hypermelanoses, [10] currently its use is restricted to vitiligo universalis for depigmentation therapy. [11]
Mechanism of action
Many theories as to how MBEH causes depigmentation have been proposed. These include the following: [12]
- MBEH reacts with tyrosinase, the key enzyme in melanin synthesis, to form a reactive quinone product. This quinone metabolite in turn binds covalently to cysteine residues in tyrosinase proteins through the sulfhydryl (-SH) group to form hapten-carrier complexes, i.e., generation of neo-antigens in the tyrosinase peptide chain occurs which excites a systemic, melanocyte destructive, inflammatory response.
- MBEH induces cellular oxidative stress in exposed pigmented cells by producing reactive oxygen species (ROS) such as peroxide. This induces lysosomal degradation of melanosomes by autophagy, in addition to disruption of melanosomal membranes and melanosome structure. This is followed by increased surface expression of melanosomal antigens by both major histocompatibility complex (MHC) class I and II routes and initiation of melanocyte Ag-specific T-cell responses.
- ROS generation also results in release of tyrosinase and MART-1 antigen containing exosomes which further contributes to immune response.
- Rapid and persistent innate immune activation also occurs in MBEH-exposed skin. MBEH is a contact-sensitizer inducing a type IV delayed type hypersensitivity response against the quinone hapten mentioned earlier. This depends on the production of pro-inflammatory cytokines such as interleukin (IL)-1b and IL-18 by the Langerhans cells or keratinocytes.
Treatment procedure
The procedure can be carried out in the privacy of one′s home and does not require frequent visits to a doctor′s office. First, an open use test should be performed on pigmented skin of the forearm. If no contact dermatitis develops, the cream can be applied to the face or areas chosen by the patient as top priority. Most patients do not treat all areas with residual pigment at once, but rather in stages, moving from highest to lowest priority. Different concentrations of MBEH can be used at one time, for example, 5% on the neck, 10% on the face, and 20% on the arms and legs. There are some patients who fail to lighten with 20% MBEH over a course of 3 to 4 months; in these patients, the concentration of MBEH can be increased to 30% and then, if there is no response over a similar time period, to 40%. Concentrations of 30% and 40% MBEH have been used primarily on the extremities, especially the elbows and knees. Concentrations greater than this are not recommended. [8]
When the vitiligo has been stable for years, a longer duration of therapy and higher concentrations of MBEH may be required. [8]
Gradually, lightening of skin occurs over a period of 4 to 12 months. [8] Depigmentation induced by MBEH is usually irreversible and histologically associated with loss of melanosomes and melanocytes. [5]
A 35 g tube of commercially prepared 20% MBEH costs around 2 500 rupees to the patient. The shelf life of the compounded product is about 6 months and it should always be kept refrigerated. [8]
Precautions
While using MBEH, a few precautions need to be kept in mind. Application of MBEH at one site can lead to loss of pigment at distant body sites, i.e., application of MBEH to the arm may result in loss of pigment on the face. [7] In majority of patients, the pigment loss is permanent. Avoid application of MBEH to the eyelids and areas close to the eye. [8] After application of MBEH, close skin-to-skin contact with another person can cause a decrease in pigmentation at the site of contact in the other person. Daily use of sunscreens with an sun protection factor (SPF) of 30 or more throughout the year is essential to prevent repigmentation as well as sunburn reactions. [7] Recurrence of pigmentation, especially in a follicular distribution, can occur spontaneously or following sun exposure over the exposed areas of face and forearms. Time to repigment may vary from as soon as therapy is discontinued [13] to months and years. [8] Repigmentation occurs in MBEH because, although it can be quite toxic to epidermal melanocytes, it often does not destroy follicular melanocytes. One theory is that there is a lack of penetration of the MBEH to the level of the hair matrices, while a second theory is that two populations of melanocytes exist in the skin, the population more resistant to vitiligo as well as to depigmentation therapy residing in the hair follicle. Hair, eyebrows, and eyelashes may be resistant to depigmentation for the same reason. [8]
Side effects
Contact dermatitis, irritant more than allergic, can develop mainly in areas of pigmented rather than vitiliginous skin. [14] If this happens, MBEH is withheld and open wet dressings are applied to the affected area along with topical steroids. When the dermatitis subsides, MBEH can be reconstituted at a lower concentration of 5%. Frequency of application is also reduced and gradually advanced as tolerated. Irritant reactions may also be decreased by mixing MBEH with emollients at the time of application. [8] Other side effects include exogenous ochronosis, [15] unmasking of telangiectasias and phlebectasias on the lower extremities, [8] pruritus, xerosis, erythema, rash, edema, conjunctival melanosis, and distant depigmentation. [7] Risk of carcinogenesis cannot be ruled out and hence it is banned from European Union since 2001 in cosmetics. [6]
How to increase the efficacy of monobenzyl ether of hydroquinone?
MBEH alone may need to be used occasionally even beyond one year to maintain depigmentation. There have been reports of MBEH therapy combined with all-trans retinoic acid (ATRA) which enhances its depigmenting and melanocytotoxic effects via inhibition of enzyme glutathione S- transferase in melanocytes. This way, we can overcome the problem of using high concentrations of 40% MBEH which can be harsh on the skin. Furthermore, retinoic acid may be substituted in future with less irritant retinoids like retinaldehyde or retinol. However, the important thing to note is that even ATRA-MBEH combination did not affect hair pigmentation in animal studies. [16]
Monomethyl Ether of Hydroquinone/4-0 Methoxyphenol
This compound is a phenol derivative and is also known as p-hydroxyanisole (HA) or mequinol. [5]
Mechanism of action
This is similar to that of MBEH. Through a dose-dependent response manner, melanocytes in the hair follicles may also be affected by 4-methoxyphenol (MP), but because of their deeper localization, these melanocytes are less susceptible to the compound compared with the epidermal melanocytes. [5]
In a recent study, depigmenting properties of 4MP and ATRA combination vs 2% 4-MP or 0.01% ATRA alone were studied on swine skin and it was found that the former produced moderate hypopigmentation in contrast to the latter which did not produce any significant hypopigmentation. However, skin treated with both returned to normal color within 7 to 12 weeks. ATRA also caused hypopigmentation by decreasing transport of melanin into keratinocytes while leaving the melanocytes intact. Hence, 4MP and ATRA affect two different stages, synthesis as well as transport of melanin. Blocking two different targets in pigmentary process leads to synergistic activity. [17]
ATRA is more active in the inhibition of melanin synthesis and tyrosinase activity of stimulated cells than non-stimulated cells; thus, hyperactive melanocytes in ultraviolet (UV) exposed skin have a greater sensitivity to the cytotoxic and inhibitory effects of 4MP and ATRA. [17]
Njoo et al. [18] in their study demonstrated total depigmentation in 69% of cases using the 4-MP cream after 4 to 12 months. In those patients who do not respond to 4-MP or who relapse after depigmenting successfully, Q-switched ruby (QSR) laser can give an additional option for complete depigmentation. [18] Advantage with 4-MP is that it can be applied on all skin types, is cheap, and easy to apply.
For resistant cases, 20% 4MP can be used after initial depigmentation with cryotherapy. Further repigmentation if occurs can be removed by single session of spot cryosurgery. Another advantage of this method is that sequential application of melanocytotoxic compounds might prevent subsequent colonization of interfollicular epidermis. [19]
Treatment procedure
The compound can be used in 20% concentration in an oil/water cream base. Patients are instructed to first apply the cream on a normal pigmented test spot (as big as 5 cm 2 ) to observe whether an allergic reaction would occur in the next 48 hours. Patients with a negative allergic reaction are allowed to apply the cream on the remaining pigmented skin areas twice daily until complete depigmentation is observed. [18] The effectiveness of 4-MP has been correlated with the duration of use of the cream; the longer the cream was used, the better the results. [5]
Side effects
Like MBEH, 4-MP also produces side effects like mild burning or itching, irregular leukoderma, contact dermatitis, ochronosis, and risk of carcinogenesis cannot be ruled out. [6] Protection from sunlight is necessary or repigmentation risk is high. [5],[6]
4-methoxyphenol vs monobenzyl ether of hydroquinone
4MP is as effective as MBEH but the side-effects like skin irritation are less common and less severe. Melanocytotoxic properties of 4MP are comparable with those of MBEH. However, compared with MBEH cream, a disadvantage of 4-MP is the longer time required prior to the onset of visible depigmentation. [5]
British guidelines in 2008 stated that depigmentation with MBEH or 4MP should be reserved for adults severely affected by vitiligo who cannot or choose not to seek repigmentation and who can accept the permanence of never tanning. The grade of recommendation was level D and the level of evidence for its use was level 4. [6]
Phenol
Phenol is inexpensive and has been used topically for chemical peelings. A nonoccluded 88% phenol application in a small area (area <20% of face or neck area) does not demand necessary cares as with Baker-Gordon′s phenol formula (40% - 50% phenol). This is because 88% phenol, being more concentrated, rapidly coagulates epidermis thus itself stopping its own further percutaneous penetration; on the other hand, Baker-Gordon′s formula, being more dilute, does not coagulate epidermis, hence showing enhanced penetration and consequently systemic absorption. [20]
Mechanism of action
All phenol compounds have toxicity over melanocytes. Transient or definite hypopigmentation is a feature of phenol cauterizing, and this is due to the development of a melanocytic incapacity to normally synthesize melanin, i.e., phenol does not cause melanocyte destruction, rather, it compromises its activity. [20] On the other hand, other depigmenting agents, such as hydroquinone and MBEH, destroy melanocytes.
Protein coagulation is observed in the epidermis immediately after application of 88% phenol solution. If phenol is re-applied, depth will be greater, with the capacity of reaching reticular dermis. [5]
Treatment procedure
Before the application, skin is cleaned with gauzes soaked with alcohol. The use of a swab moistened with phenol is used to treat small areas, till cutaneous frosting occurs. The patient feels a burning sensation for approximately 60 seconds, which gradually decreases in intensity and it can last from minutes to hours. After two sessions, 45 days apart, total elimination of residual pigmented areas is noticed. No signs of repigmentation have been seen till after one and a half year of therapy. [20]
Post-procedure care
Delicate cleaning with saline, use of antibiotic ointment with steroids of mild to moderate potency and sun blocks should be done. Antiviral use is indicated for patients with a history of herpes simplex.
Side effects
Generally, 88% phenol solution does not produce any complications in experienced hands. However, sometimes 88% phenol solution produces complications such as non-aesthetic scar formation, dyschromia, and development of herpetic eczema. High-dose phenol usage is toxic, so it should not be applied over large areas. Phenol exerts a marked corrosive action on any tissue it comes in contact with, be it upon ingestion, inhalation, or direct contact with the skin. Its cellular uptake is both rapid and passive because of its lipophilic character and signs of systemic toxicity develop soon after exposure. Phenol′s main target organs are liver, kidney, respiratory and cardiovascular systems. Cardiovascular shock, cardiac arrhythmias, and bradycardia, as well as metabolic acidosis have been reported within 6 hours of skin-peeling procedures with phenol. Repigmentation may occur if patients do not protect themselves properly from ultraviolet radiation. [20]
88% phenol vs monobenzyl ether of hydroquinone
With phenol, the side effects are lesser. It can be used in areas where MBEH is not available. It is a cheap, practical product, with no complications in experienced hands. [20]
Laser Therapy
Recently, many lasers have been advocated for depigmentation therapy in vitiligo. The main advantages include that they are an effective, fast, and safe method with short treatment duration. They are more effective in vitiligo patients with a positive Koebner phenomenon. [21] Lasers can be used in cases with failure to MBEH/other bleaching agents and for areas like face where rapid depigmentation is required within days. Lasers also overcome the disadvantages of topical therapies, e.g., local redness, burning, itching; also, topical therapy takes a long time of approx 10 months to depigment with the possibility of only partial depigmentation and a relatively high failure rate, and repigmentation may occur. With lasers, large areas can be depigmented in one sitting, as opposed to depigmentation performed using a bleaching agent. An additional advantage is that the risk of scar formation is less. [18]
Q-switched ruby (QSR) laser (694 nm)
Successful use of QSR laser was shown by Njoo et al. [18] for depigmentation in vitiligo. Bigger confluent areas of pigment on the extremities can first be treated with a topical therapy. Combination therapy was seen to give better results than with any of the method alone. Depigmentation is rapid and occurs by 1 to 2 weeks.
Mechanism of action: Lasers have proven to be highly effective in selectively targeting melanocytes for destruction, thus causing depigmentation. The QSR lasers are known to induce selective photothermolysis of pigmented lesions because their wavelengths are between 600 nm and 800 nm, which are absorbed easily by melanin. Because the duration of the energy pulse is shorter than the thermal relaxation time of melanosomes, no energy (i.e., heat) will be transduced into the surrounding tissue. [5]
Treatment procedure: This laser emits pulses with a wavelength of 694 nm with a pulse duration between 25 and 28 nanoseconds in a frequency of 1 to 1.2 Hz. [18] With a 5-mm spot size, the laser is capable of administering pulses with an energy intensity between 0 and 10 J/cm 2 . However, the energy can be varied from 10 to 40 J/cm 2 according to the skin type. [21] First, a test spot of 5 cm 2 is treated and then evaluated after 8 weeks to see whether clinical depigmentation has occurred. If depigmentation is evident, further laser treatment is performed until total depigmentation occurs. If no sign of depigmentation is seen, further laser treatment is withheld. To avoid pain, the procedure is performed under eutectic mixture of lidocaine (EMLA), 25 mg/g and prilocaine 25 mg/g. A maximum size of 80 cm 2 can be treated in each session. Treated skin is covered with sterile gauze and patients are told to avoid exposure to sunlight for 6 weeks. Multiple treatments are performed at an interval of 2 to 4 weeks to bring about complete depigmentation involving larger areas. [18]
Tanning of skin prior to using Q-switched ruby laser
Tanning can induce activation of melanocytes in normal pigmented areas and these activated melanocytes are then the target of selective photothermolysis. Therefore, QSR laser therapy after tanning can induce permanent damage in activated melanocyte-containing structures. In a study by Kim et al., [22] no repigmentation was seen till one year when tanning was employed prior to using QSR laser.
Q-switched alexandrite laser (755 nm)
Rao and Fitzpatrick [23] reported the use of alexandrite laser in a 68-year-old woman in whom 18 sessions of QSR laser over 5 years along with 20% MBEH application had failed in clearing residual pigmentation. Within 3 months after each laser session, most treated areas would begin to repigment. Subsequently, Q-switched alexandrite (QSA) laser (755 nm, 50-100 ns) was tried. A total of 10 treatment sessions (mean per session, 3.4-6.0 J/cm 2 , 3-4 mm, 484-1636 pulses) were administered to the recalcitrant pigmented patches. Topical MBEH therapy was discontinued on laser-treated sites. Within 22 months, the patient was nearly clear of all pigment within treated sites. At 12 months of follow-up, she had only minimal recurrence of pigment.
The QSA laser is advantageous over the QSR laser because it has a faster pulse frequency, which allows for more rapid therapy. In addition, it also has a higher wavelength of 755 nm, as compared with the 694 nm QSR laser, which facilitates greater tissue penetration and improves results. [23]
Other potential Q-switched lasers that can selectively destruct melanocytes include neodymium: yttrium aluminium garnet (Nd: YAG) laser (1064 nm) and the frequency-doubled Nd:YAG laser (532 nm). [5]
Side effects: The main disadvantage is that local anesthesia is required as the procedure is painful. Treatment is only possible in clinic, rendering it an expensive therapy. The QSR laser therapy may fail in permanently removing pigmented patches and after several months of treatment, follicular repigmentation may develop due to migration of perifollicular melanocytes to the epidermis, indicating that these are not destroyed by the laser therapy. The depigmenting effect of the QSR laser therapy can be considered as a Koebner phenomenon. Thus, patients with active vitiligo may respond better to laser therapy than those with stable disease. Also, patients with negative Koebner may relapse. [18]
Cryotherapy
When rapid depigmentation is desirable, physical agents like cryotherapy and lasers work faster than bleaching agents. Radmanesh [24] has demonstrated the use of cryosurgery for cost-effective rapid depigmentation that is permanent and has excellent cosmetic result but only over limited areas at a time.
Mechanism of action
Vitiligo patches with positive Koebner respond well to cryosurgery. Intracellular ice formation leads to irreversible tissue damage. In this aspect, melanocytes are more sensitive to cryodamage in comparison with other epidermal cells. The degree of damage depends on the rate of cooling and minimum temperature achieved. Inflammation develops within 24 hours of treatment, further contributing to destruction of lesion through immunologically mediated mechanisms. Mild freezing leads to a dermoepidermal separation, which is useful in treating epidermal lesions.
Treatment procedure
Spot testing by a single freeze-thaw cycle is done and when the edema and erythema subside, the patches are treated with cryotherapy 3 to 6 weeks later. Both CO 2 and liquid N 2 can be used. A 2-cm flat-topped and round cryoprobe is held approx 40 mm from the skin surface. The whole patch can be frozen with a single freeze-thaw cycle from the periphery and then by forming successive rows inward. Procedure should be terminated when a narrow (< 1 mm) frost rim forms around the periphery of the cryoprobe. The rim can develop within 10 to 20 seconds by a cryogun connected to a container with barometric pressure above 80 kg/cm 2 . For lesions around the orbits or uneven areas of the nose, cyoprobes with smaller diameters may be required. No more than one freeze-thaw cycle is advised. However, another study has used two freeze-thaw cycles also. [19]
After a week, a depigmented, un-scarred, slightly atrophic, and erythematous smooth area appears. The best cosmetic result is obtained 4 weeks after cryotherapy. The depigmentation is permanent, although more than one session may be required for partially depigmented lesions, with 4 to 6 weeks intervals, before complete depigmentation occurs. [24]
In those with more extensive pigmented patches, cryotherapy can be performed in several sessions 1 to 3 weeks apart in order to prevent discomfort to the patient. Spot cryotherapy can be used for areas which repigment.
Advantage
Cryotherapy has been suggested to depigment MBEH-resistant skin. The procedure requires no anesthesia and can be performed in an outpatient department. No dressing, sedatives or antibiotics are required. Preparation time is short and inexpensive. The risk of infection is low and wound care is minimal. This method is simple, easy to perform, safe, efficacious, and cost effective. Depigmentation developed by cryotherapy is permanent and without scarring if performed by experienced dermatologists. Many patients prefer a single short-term procedure than applying an expensive compound for 10 months or more with unpredictable effects and a considerable failure rate.
Disadvantage
A qualified experienced person is required for this and hence treatment is hospital based. Also, cryotherapy is suitable for small lesions and a single sitting cannot be utilized for depigmenting extensive areas unlike lasers.
Side effects
Immediate side effects include edema, pain, and bulla formation. If cryotherapy is performed aggressively, it can lead to permanent scarring. Cryotherapy should be used by experienced person.
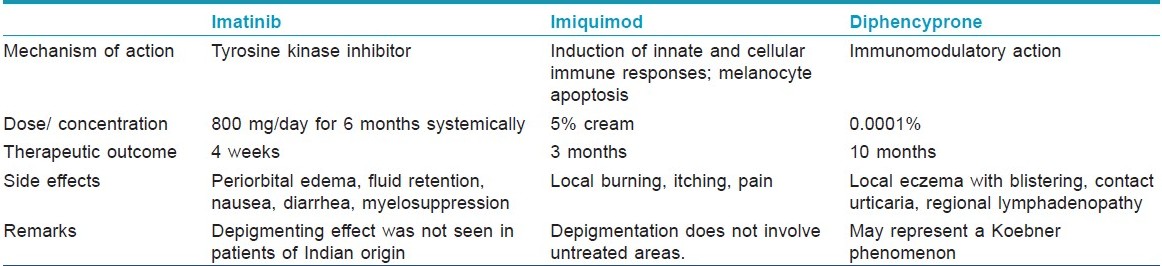
Imatinib
Leong et al.[25] first reported 13 patients of chronic myeloid leukemia receiving imatinib mesylate for 2 to 3 months, who gradually developed skin depigmentation (whitening/generalized hypopigmentation). Skin became darker during the discontinuation period and began lightening again once imatinib mesylate treatment was resumed.
Mechanism of action
It is postulated that imatinib mesylate, being a tyrosine kinase inhibitor, may interfere with the production of melanin, resulting in decreased pigmentation of the skin. After patients begin receiving imatinib mesylate, hypopigmentation can be observed within 12 weeks. It is difficult to define the onset exactly, because the change is gradual. An ethnic and/or genetic basis has also been considered. [25]
Side effects
The side-effects of imatinib mesylate are periorbital edema, fluid retention, weight gain, musculoskeletal pain, headache, nausea, diarrhea, and myelosuppression. In addition, a number of dermatological side-effects have been documented, such as follicular mucinosis, erythroderma, and lichenoid eruption. Imatinib mesylate can also induce local or generalized hyperpigmentation. Severe congestive cardiac failure is an uncommon but recognized side effect of imatinib. Imatinib in children can delay normal growth, although a proportion will experience catch-up growth during puberty. [5]
Imiquimod
Imiquimod is a novel imidazoquinoline immune response modifier, frequently used for topical treatment of anogenital warts and basal cell carcinomas. [1]
Mechanism of action
Imiquimod increases production of proinflammatory cytokines, mainly interferon (IFN)-α, tumor necrosis factor (TNF)-α, and IL-6, IL-8, IL-10, IL-12, all of which augment the type 1 helper T-cell (TH1) response which is found to be prominent in the pathogenesis of vitiligo. [5] Imiquimod also stimulates CD8 cells to become cytotoxic and enhances antigen presentation. [26] Recently, it was reported that human melanocytes express toll like receptor 7 (TLR7). When applied topically, imiquimod binds to TLR7 followed by stimulation of various cytokines which induce the above mentioned T lymphocytic response. [27] Imiquimod also has a direct action on melanocytes via apoptosis of melanocytes. This action is related to reduction of expression of Bcl-2 and/or an increase in the proapoptotic stimulus (cytotoxic T lymphocytes, natural cytotoxic T cells/killer cells, granzymes B, Fas, TNF, Bax, etc.). [28]
Therefore, it is possible that imiquimod may cause elimination of melanocytes by direct influence on cells as well as inducing acquired immunity indirectly, thus inducing vitiligo like hypopigmented lesions.
Method of application
5% imiquimod use may be followed by erythema which gradually turns to depigmented patches over a period of 3 months. No repigmentation has been seen till 6 months after the depigmentation. Also, depigmentation did not extend to areas that had not been treated with imiquimod. [29]
Side effects
The most common side-effects of imiquimod are burning, itching, pain, erythema, erosions, and scabbing/crusting at the target site which occur more frequently with twice-daily application. [5]
Diphencyprone
Topical application of diphencyprone (DPCP) when used for the treatment of alopecia areata was found to produce depigmentation as part of its side-effects. Duhra and Foulds [30] reported a case of alopecia totalis in whom sensitization therapy with topical DPCP was commenced. There was marked reaction with erythema and edema on the forearm after 3 days, but the scalp manifested only slight macular erythema. The forearm reaction subsided after 2 weeks and was replaced 6 weeks later by a depigmented patch. Similar depigmented areas appeared on the nape of the neck and the midline of the back. These remained unchanged for 2 years after discontinuing DPCP therapy. Electron microscopy and incubation with dopa in affected skin revealed an absence of melanosomes and melanocytes.
DPCP-induced vitiligo is rare and may represent a Koebner phenomenon in predisposed individuals. Vitiligo can develop even with DPCP concentrations as low as 0.0001%. [30]
Side effects
Adverse effects include local eczema with blistering, regional lymphadenopathy, hyperpigmentation, hypopigmentation, and vitiligo. [1]
The depigmenting effects of imatinib and imiquimod have only been reported in few studies and randomized control trials are lacking. Hence, further studies are required on these agents and other similar molecules before they can be used as mainstream depigmenting agents.
Experimental Agents
A huge number of phenolic compounds have been tested as inhibitors of melanin synthesis. In one animal study, eight compounds were selected on the basis of their known depigmenting effects, including hydroquinone (H), 4-ethoxyphenol (4-EP), 4-methylcatechol (4-MC), 4-tert-butylcatechol (4-t-BC), monobenzone (M), hydroquinone bis (2-hydroxyethyl)-ether (HHEE), and catechol (C). These compounds were injected into animal skin as 10% and 20% solutions dissolved in 95% ethanol. Six of the eight compounds tested showed positive depigmenting effects at 10% except C and HHEE. Compounds showed increased necrosis at a concentration of 20%. [31]
Both 4-MC and 4-MP were also applied topically in a cream (liposome) base onto the same animal in a rub-in form but failed to cause any depigmentation, which was attributed to failure of the drug to penetrate the horny layer of the skin and low absorption rates due to drug remaining in the liposome.
Hydroquinone in higher concentrations of more than 4%, IFN-gamma, and alkylating agent busulfan should be further investigated as topical depigmenting agents for use in human beings with vitiligo universalis. Some compounds like N-acetyl-4-S-cysteaminylphenol, N-2,4-acetoxyphenyl thioethyl acetamide and N-hydroxycinnamoylphenalkylamides have been proposed as anti-melanoma drugs as well as for use as hypopigmenting agents. Vaccines using melanoma-associated antigen were reported by many authors to produce depigmentation by eliciting an autoimmune response directed against malignant but also normal melanocytes. 4-(p-hydroxyphenyl)-2-butanone and 4-n-butylresorcinol not only inhibit tyrosinase but also act as cytotoxic agents as they are oxidized to their quinonic forms leading to a double effect. Ethanolic extracts of Myrica rubra dried leaves have shown good depigmenting effects in vitro and pseudo superoxide dismutase activity. They contain quercetin, myricetin, and some 3-O-ramnosides derivatives. In vivo studies have been recommended to evaluate their potential use as depigmenting agents. [32]
To conclude, depigmentation therapies are the last resort for extensive vitiligo cases. These therapies are evolving and new therapies have come in vogue apart from MBEH and 4 MP. In future, some experimental products may become mainstay of depigmentation therapies.
| 1. |
Halder RM, Taliaferro SJ. Vitiligo. In: Wolff K, Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Lefell DJ, editors. Fitzpatrick's Dermatology in General medicine. 7 th ed, Vol 1. New York: McGraw Hill; 2008. p. 616-22.
th ed, Vol 1. New York: McGraw Hill; 2008. p. 616-22.'>[Google Scholar]
|
| 2. |
Grimes PE. White patches and bruised souls: Advances in the pathogenesis and treatment of vitiligo. J Am Acad Dermatol 2004;S1:S5-7.
[Google Scholar]
|
| 3. |
Ongenae K, Beelaert L, Van Geel N, Naeyaert JM. Psychosocial effects of vitiligo. J Eur Acad Dermatol Venereol 2006;20:1-8.
[Google Scholar]
|
| 4. |
Parsad D, Dogra S, Kanwar AJ. Quality of life in patients with vitiligo. Health Qual Life Outcomes 2003;1:58.
[Google Scholar]
|
| 5. |
Alghamdi KM, Kumar A. Depigmentation therapies for normal skin in vitiligo universalis. J Eur Acad Dermatol Venereol 2011;25:749-57.
[Google Scholar]
|
| 6. |
Gawkrodger DJ, Ormerod AD, Shaw L, Mauri-Sole I, Whitton ME, Watts MJ, et al. Guideline for the diagnosis and management of vitiligo. Br J Dermatol 2008;159:1051-76.
[Google Scholar]
|
| 7. |
Drake LA, Dinehart SM, Farmer ER,Goltz RW, Graham GF, Hordinsky MK, et al. Guidelines of care for vitiligo. J Am Acad Dermatol 1996;35:620-6.
[Google Scholar]
|
| 8. |
Bolognia JL, Lapia BK, Somma S. Depigmentation therapy. Dermatol Ther 2001;14:29-34.
[Google Scholar]
|
| 9. |
Oliver EA, Schwartz L, Warren LH. Occupational leukoderma. JAMA 1939;113:927-8.
[Google Scholar]
|
| 10. |
Lerner AB, Fitzpatrick TB. Treatment of melanin hyperpigmentation. JAMA 1953;152:577-82.
[Google Scholar]
|
| 11. |
Mosher DB, Parrish JA, Fitzpatrick TB. Monobenzylether of hydroquinone: A retrospective study of treatment of 18 vitiligo patients and a review of the literature.Br J Dermatol 1977;97:669-81.
[Google Scholar]
|
| 12. |
Van doon Boorn JG, Melief CJ, Luiten RM. Monobenzone induced depigmentation: From enzymatic blockade to autoimmunity.Pigment Cell Melanoma Res 2011;24:673-9.
[Google Scholar]
|
| 13. |
Oakley A. Rapid repigmentation after depigmentation therapy: Vitiligo treated with monobenzyl ether of hydroquinone. Australas J Dermatol 1996;37:96-8.
[Google Scholar]
|
| 14. |
Lyon CC,Beck MH.Contact hypersensitivity to monobenzyl ether of hydroquinone used to treat vitiligo.Contact Dermatitis1998;39:132-56.
[Google Scholar]
|
| 15. |
Charlin R, BarcauiCB, Kac BK, Soares DB, Rabello Fonseca R, Azulay-Abulafia L. Hydroquinone induced exogenous ochronosis: A report of four cases and usefulness of dermoscopy. Int J Dermatol 2008;47:19-23.
[Google Scholar]
|
| 16. |
Kasraee B, Fallahi MR, Ardekani GS, Doroudchi G, Omrani GR, Handjani S, et al. Retinoic acid synergistically enhances the melanocytotoxic and depigmenting effects of MBEH in black guinea pigs skin. Exp Dermatol 2006;15:509-14.
[Google Scholar]
|
| 17. |
Nair X, Parab P, Suhr L, Tramposch KM. Combination of 4-hydroxyanisole and all-trans retinoic acid produces synergistic skin depigmentation in swine. J Invest Dermatol 1993;101:145-9.
[Google Scholar]
|
| 18. |
Njoo MD, Vodegel RM, Westerhof W. Depigmentationtherapy in vitiligo universalis with topical 4-methoxyphenol and the Q-switched ruby laser. J Am Acad Dermatol 2000;42:760-9.
[Google Scholar]
|
| 19. |
Di Nuzzo S, Masotti A. Depigmentation therapy in vitiligo universalis with cryotherapy and 4-hydroxyanisole. Clin Exp Dermatol 2010;35:215-6.
[Google Scholar]
|
| 20. |
Zanini M. Depigmentation therapy for generalized vitiligo with topical 88% phenol solution. An Bras Dermatol 2005;80:415-6.
[Google Scholar]
|
| 21. |
Thissen M, Westerhof W. Laser treatment for further depigmentation in vitiligo. Int J Dermatol 1997;36:386-8.
[Google Scholar]
|
| 22. |
Kim YJ, Chung BS, Choi KC. Depigmentation therapy with Q-switched ruby laser after tanning in vitiligo universalis. Dermatol Surg 2001;27:969-70.
[Google Scholar]
|
| 23. |
Rao J, Fitzpatrick RE. Use of the Q-switched 755 nm alexandrite laser to treat recalcitrant pigment after depigmentation therapy for vitiligo. Dermatol Surg 2004;30:1043-5.
[Google Scholar]
|
| 24. |
Radmanesh M. Depigmentation of the normally pigmented patches in universal vitiligo patients by cryotherapy. J Eur Acad Dermatol Venereol 2000;14:149-52.
[Google Scholar]
|
| 25. |
Leong KW, Lee TC, Goh AS. Imatinib mesylate causes hypopigmentation in the skin. Cancer 2004;100:2486-7.
[Google Scholar]
|
| 26. |
Zirvi TB, Costarelis G, Gelfand JM. Vitiligo-like hypopigmentation associated with imiquimod treatment of genital warts. J Am Acad Dermatol 2005;52:715-6.
[Google Scholar]
|
| 27. |
Kang HY, Park TJ, Jin SH. Imiquimod, a toll-like receptor 7 agonist, inhibits melanogenesis and proliferation of human melanocytes. J Invest Dermatol 2009;129:243-6.
[Google Scholar]
|
| 28. |
Kim CH, Ahn JH, Kang SU, Hwang HS, Lee MH, Pyun JH, et al. Imiquimod induces apoptosis of human melanocytes. Arch Dermatol Res 2010;302:301-6.
[Google Scholar]
|
| 29. |
Senel E, Seckin D. Imiquimod-induced vitiligo-like depigmentation. Indian J Dermatol Venereol Leprol 2007;73:422-3.
[Google Scholar]
|
| 30. |
Duhra P, Foulds IS. Persistent vitiligo induced by diphencyprone. Br J Dermatol 1990;123:415-6.
[Google Scholar]
|
| 31. |
Schwartzkopf KS, Stookey JM, Hull PR, Clark EG. Screening of depigmenting compounds for the development of an alternative method of branding beef cattle. J Anim Sci 1994;72:1393-8.
[Google Scholar]
|
| 32. |
Solano F, Briganti S, Picardo M, Ghanem G. Hypopigmenting agents: An updated review on biological, chemical and clinical aspects. Pigment Cell Res 2006;19:550-71.
[Google Scholar]
|
Fulltext Views
23,536
PDF downloads
3,604





