Translate this page into:
Dermatological adverse reactions to cancer chemotherapy
Correspondence Address:
Srinath M Kambil
Department of Dermatology, Father Muller Medical College, Kankanady, Mangalore - 575 002, Karnataka
India
| How to cite this article: Pavey RA, Kambil SM, Bhat RM. Dermatological adverse reactions to cancer chemotherapy. Indian J Dermatol Venereol Leprol 2015;81:434 |
INTRODUCTION
New anticancer drugs developed over the last few decades have improved the survival rate in cancer patients. Traditional chemotherapy drugs as well as the newer targeted agents are associated with a wide array of cutaneous toxicities. [1] Toxic effects on skin, hair and nails can negatively affect the quality of life and also lead to interruption or discontinuation of these drugs. [2] The aim of our study was to assess the mucocutaneous adverse effects of both single and combined chemotherapy regimens in cancer patients.
Materials and Methods
A total of 53 patients attending the oncology outpatient department or those admitted in the oncology ward of Father Muller Medical College Hospital, Mangalore between October 2012 and September 2013 were prospectively studied after obtaining ethical clearance and the cutaneous adverse effects related to chemotherapy were noted. We included patients of both sexes who suffered from mucocutaneous adverse effects which began after initiation of the anti-cancer drug. Exclusion criteria included patients developing cutaneous manifestations as a result of internal malignancies, patients who already had muco-cutaneous symptoms at the start of therapy, and those on radiotherapy.
RESULTS
Out of the 53 cancer patients studied, 19 patients were on a single chemotherapy drug and 34 were on combined chemotherapy. The various drugs used in the study were: cetuximab, geftinib, imatinib, sorafenib, paclitaxel, vincristine, vinblastine, 6-mercaptopurine, 5-fluorouracil, cytarabine, capecitabine, gemcitabine, cisplatin, carboplatin, oxaliplatin, etoposide, cyclophosphamide, doxorubicin, daunorubicin, epirubicin, hydroxyurea, CHOP (cyclophosphamide, doxorubicin, vincristine, prednisolone), and ABVD (doxorubicin, bleomycin, vinblastine, dacarbazine) regimens.
Breast cancer was the most common cancer seen in 12 patients followed by ovarian cancer in 8 patients, acute lymphocytic leukemia in 7 patients and lung cancer in 6 patients. The remaining types are depicted in [Figure - 1].
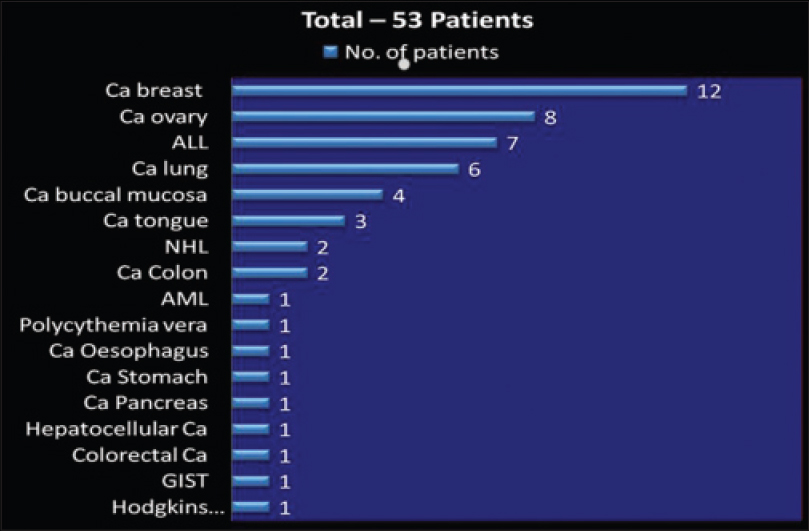 |
| Figure 1: Types of cancer in the 53 patients (N = 53). Ca:Carcinoma, ALL: Acute lymphatic leukemia, NHL: Non hodgkins lymphoma, AML: Acute myeloid leukemia, GIST: Gastrointestinal stromal tumour |
Of the 53 patients, 25 (47%) were males with a mean age of 46.9 years and 28 (53%) were females with a mean age of 47.4 years. Nail changes were the most common adverse effect noticed in 33 (62.2%) patients, followed by hair changes in 20 (37.7%), skin changes in 19 (33.9%) and mucosal changes in 2 (3.7%) patients [Figure - 2].
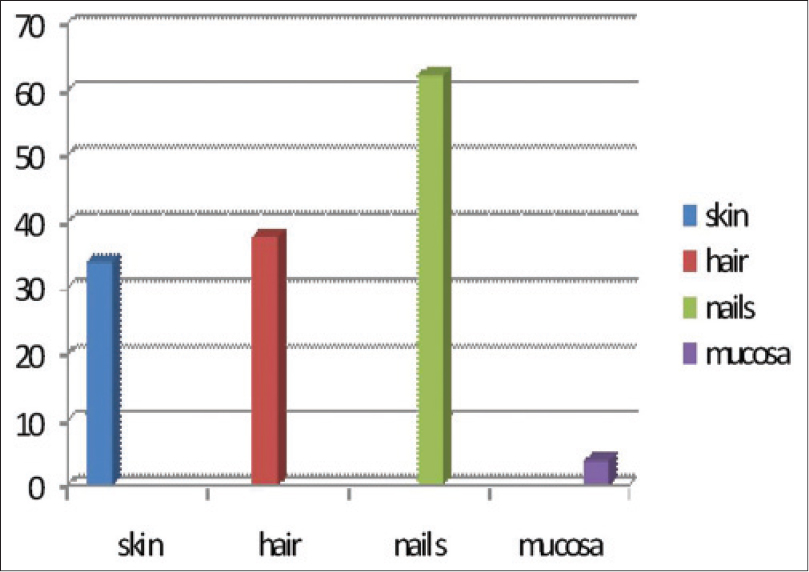 |
| Figure 2: Frequency of adverse effects |
The skin changes were acneiform (papulopustular) rash in 5 (27.7%) patients, xerosis in 4 (22.2%), hyperpigmentation in 4 (22.2%), and toxic epidermal necrolysis, hand foot syndrome, extravasation, erythema nodosum and supravenous hyperpigmentation in 1 patient each [Table - 1].
The most common nail finding observed was melanonychia which was seen in 26 (78.7%) patients, followed by Muehrcke′s lines, Mee′s lines, and Beau′s lines [Table - 2].
Hair changes were mainly in the form of anagen effluvium seen in 20 (37.7%) patients. Mucosal changes included pigmentation of tongue and stomatitis [Table - 3].
DISCUSSION
Anti-cancer drugs usually affect rapidly growing cells and hence, the skin, hair follicles and nail matrix are the frequent targets of their toxicities. [3] Various anti-cancer drugs such as epidermal growth factor receptor (EGFR) inhibitors including cetuximab and geftinib, multikinase inhibitors (imatinib, sorafenib), taxanes (paclitaxel), vinca alkaloids (vincristine, vinblastine), antimetabolites (6-mercaptopurine, 5-fluorouracil, cytarabine, capecitabine, gemcitabine), genotoxic agents (cisplatin, carboplatin, oxaliplatin, etoposide, cyclophosphamide, and anthracyclines like doxorubicin, daunorubicin, epirubicin), hydroxyurea, cyclophosphamide, doxorubicin, vincristine, prednisolone (CHOP) and ABVD (doxorubicin, bleomycin, vinblastine, dacarbazine) regimens are associated with prominent and sometimes dose-limiting dermatologic complications. [1]
Epidermal growth factor receptor inhibitors are associated with intensely itchy papulo-pustular rash or acneiform eruptions that occur mainly on the seborrheic areas such as the face, scalp and chest. [4] It is hypothesized that the action of the drug alters signaling pathways resulting in keratinocyte growth arrest, apoptosis, decreased cell migration and increased differentiation and elicits an inflammatory response mediated by various cytokines released from keratinocytes. [5] In our study, four patients on these drugs had an itchy papulo-pustular rash over seborrheic areas [Figure - 3].
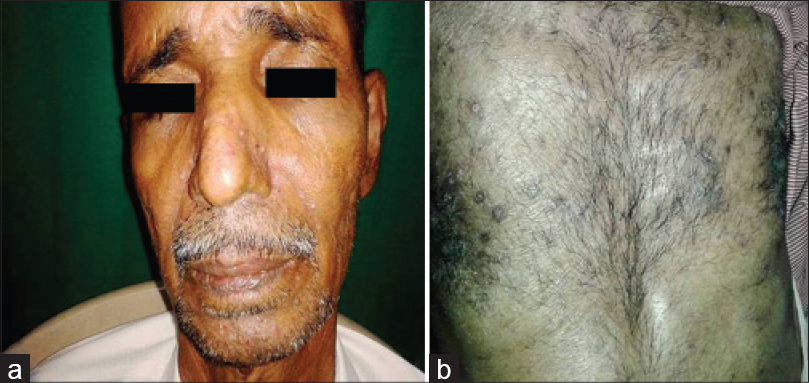 |
| Figure 3: Papulopustular rash due to cetuximab (a) and gefitinib (b) |
Hand-foot syndrome represents the clinically significant and occasionally dose-limiting skin toxicity of cytarabine, doxorubicin, 5-fluorouracil, sorafenib, and sunitinib. Dual inhibition of vascular endothelial growth factor receptor (VEGFR) and platelet-derived growth factor receptor (PDGFR) disrupts the normal repair process involving capillaries and fibroblasts. This blockade, in combination with repeated subclinical trauma and friction to areas such as palms and soles, leads to inflammation. It usually presents as painful symmetric erythema over the thenar or hypothenar eminences and pad of distal phalanges and less often on the soles. In severe cases, blistering develops over the swollen erythematous areas. [1],[5],[6],[7] In our study, a single case of hand-foot syndrome was seen in a patient on sorafenib which occurred during the first cycle of treatment [Figure - 4]. The patient was treated with cold compresses, emollients and topical steroids, and the drug was temporarily stopped. The drug was restarted at slightly lower dose once the skin lesions improved.
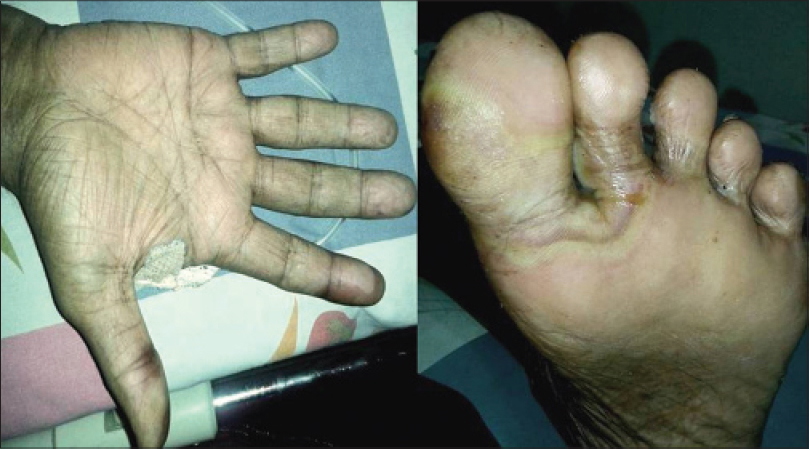 |
| Figure 4: Hand foot syndrome due to sorafenib |
Hyperpigmentation has been reported to occur with anti-cancer drugs, which may be in the form of diffuse or localized involvement of skin, mucosa or nails. The mechanism remains unknown, but it is postulated to be due to accumulation of drug in skin or a direct toxic effect on melanocytes stimulating increased melanin production or elevated adrenocorticotropic hormone and melanocyte stimulating hormone. The drugs commonly causing pigmentation are cyclophosphamide, hydroxyurea, doxorubicin, cisplatin, fluorouracil, etoposide, busulfan, and bleomycin. [1] Imatinib usually produces pigmentary dilution of skin but rarely, can cause paradoxical hyperpigmentation of skin, hair and nails. [6],[8],[9] In our study, pigmentation was seen in patients treated with hydroxyurea, imatinib, capecitabine and the combination of etoposide and paclitaxel. Supravenous hyperpigmentation was seen in a case of Hodgkin′s lymphoma on ABVD (doxorubicin, bleomycin, vinblastine, dacarbazine) regimen 3 months after the onset of treatment [Figure - 5]. Similar findings were seen in a case reported by Baselga et al., after 6 months of treatment and the drugs responsible were thought to be bleomycin and doxorubicin. [10] Pigmentation of tongue was seen in a single patient on hydroxyurea [Figure - 6]. Hydroxyurea can produce diffuse pigmentation of face, neck, palms with accentuation in areas of pressure, longitudinal and transverse bands or diffuse nail pigmentation and patchy pigmentation of tongue and buccal mucosa. [1],[11],[12]
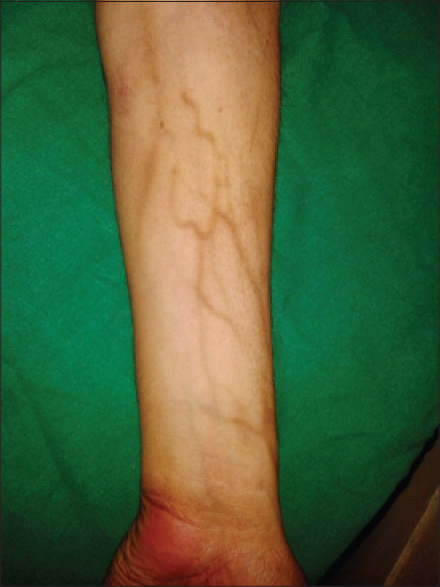 |
| Figure 5: Supravenous pigmentation in a patient on doxorubicin, bleomycin, vinblastine, dacarbazine (ABVD) regimen |
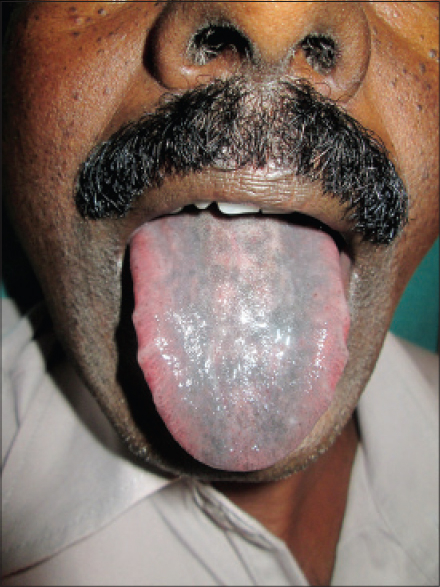 |
| Figure 6: Pigmentation of tongue due to hydroxyurea |
One case of extravasation was seen in a patient on paclitaxel and carboplatin [Figure - 7]. Both drugs are documented to be irritant agents and cause burning, warmth, erythema and tenderness in the extravasated area with occasional necrosis. [13] In our case, both drugs were stopped temporarily and cold compresses and limb elevation were undertaken. A single case of toxic epidermal necrolysis due to capecitabine was seen in our study, which was also reported by Matos-Fernandez et al. [14] In our case, the drug was completely stopped.
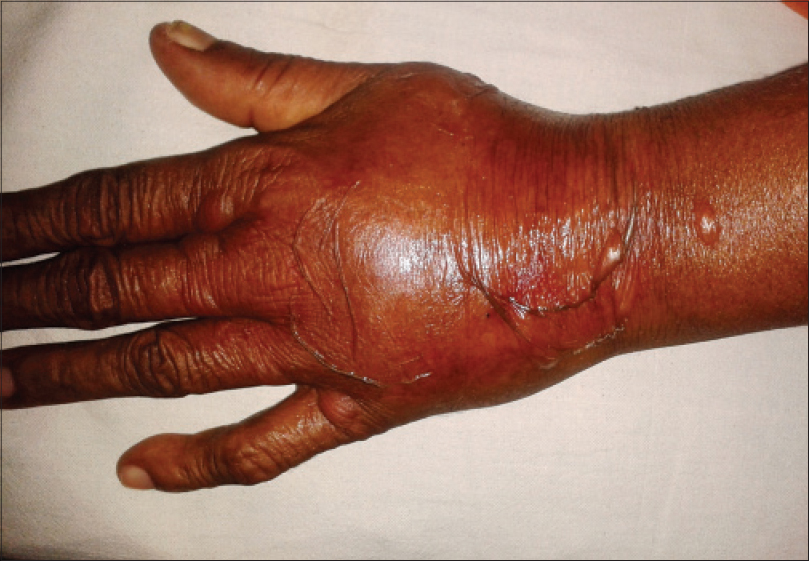 |
| Figure 7: Extravasation reaction in a patient on paclitaxel and carboplatin |
Nail matrix cells are continuously dividing cells that are frequently affected by chemotherapy leading to cosmetic nail disfigurement. [15] Nail changes were the most prevalent adverse effect in our study [33 (62.2%) patients]. Nail hyperpigmentation [Figure - 8] in the form of diffuse and transverse pigmentation was the most common nail change seen in 26 (78.7%) patients and these patients were on combined regimens. Melanonychia is usually caused by matrix melanin distribution in the growing nail plate. Vincristine, doxorubicin, hydroxyurea, bleomycin, cyclophosphamide, daunorubicin, dacarbazine, and 5-fluorouracil have been reported to cause melanonychia in previous studies and similar findings were seen in our study. [3],[16] Muehrcke′s lines [Figure - 9] were seen in four patients, which was the second most common nail change in our study. A study done in children on cancer chemotherapy at Chang Gung Memorial Hospital in Taiwan showed Muehrcke′s lines to be the commonest nail change. The exact pathogenesis is unknown but the suggested reasons are edema of the nail bed, which occurs due to hypoalbuminemia, and an alteration of nail plate attachment to the nail bed, which occurs due to vascular compromise following chemotherapy. [15] Serum albumin was normal in all four patients. Vincristine, cyclophosphamide, and doxorubicin are associated with Mee′s lines which is due to sudden direct toxicity on the nail matrix. [17] Two patients of non-Hodgkin lymphoma (NHL) on cyclophosphamide, doxorubicin, vincristine, prednisolone (CHOP) regimen had Mee′s lines [Figure - 10].
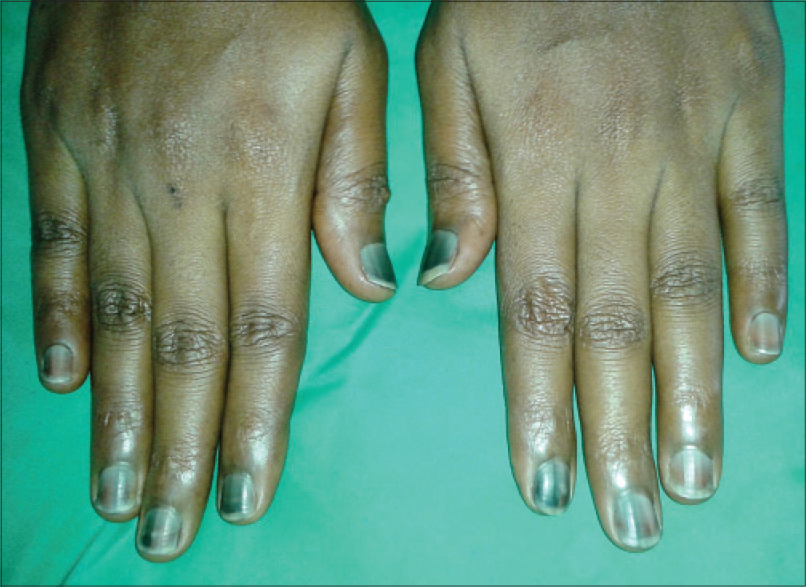 |
| Figure 8: Nail hyperpigmentation in a patient on vincristine and daunorubicin |
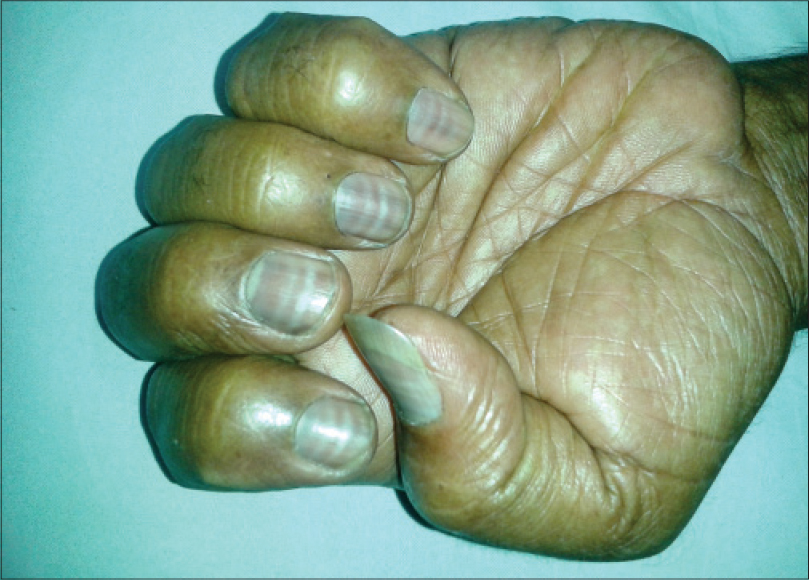 |
| Figure 9: Muehrcke's lines in a patient on epirubicin and oxaliplatin |
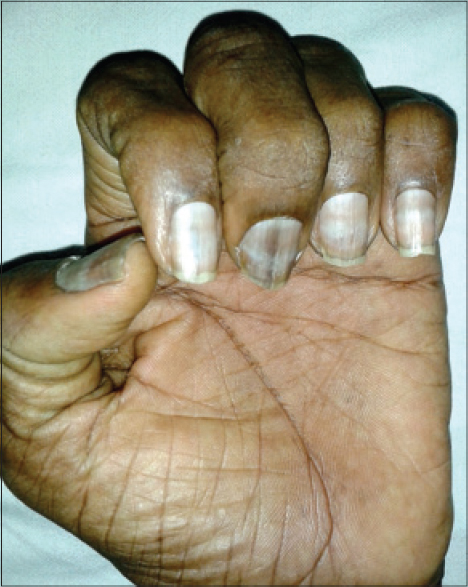 |
| Figure 10: Mee's lines in a patient on cyclophosphamide, doxorubicin, vincristine, prednisolone (CHOP) regimen |
Hair loss has been rated as one of the most distressing side effects of chemotherapy, along with vomiting and nausea. [18] There have been reports of refusal of chemotherapy, especially among women, because of the risk of hair loss. Alopecia was the second most common adverse effect in our study [20 (37.7%) patients]. However, alopecia was the most common adverse effect in a study done by Kamil et al. and Chewchanvit et al. [3],[19] Hair loss was seen within the first 1 month after the onset of chemotherapy in our study, which was also observed in a study by Chadha et al. [20] Anagen effluvium is the most common cause of hair loss associated with anti-cancer drugs and it usually begins in 1-2 weeks after starting the drug, becoming more apparent in the subsequent 4-8 weeks. Anagen effluvium was the likely cause of hair loss in all our patients [Figure - 11]. Drugs like doxorubicin, daunorubicin, docetaxel, and cyclophosphamide are more likely to cause anagen effluvium. [16] In our study, hair loss was seen in patients on paclitaxel and carboplatin, paclitaxel and daunorubicin, cyclophosphamide and daunorubicin, and vincristine and daunorubicin, as noted in previous reports.
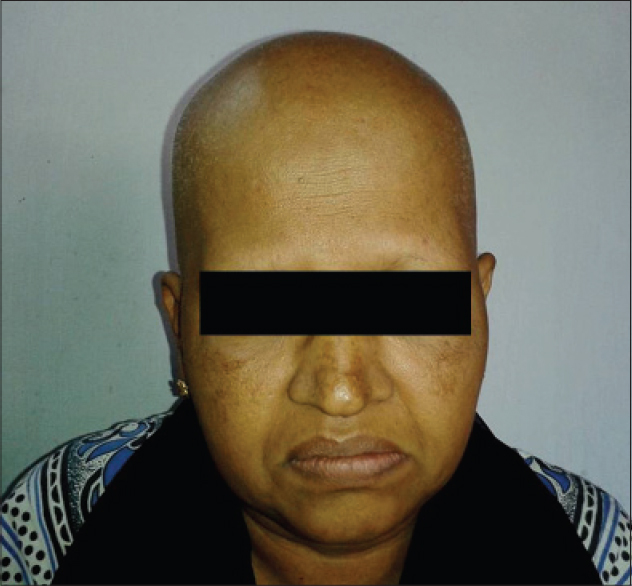 |
| Figure 11: Anagen effluvium in a patient on paclitaxel and carboplatin |
Although both the newer targeted therapies and the traditional anticancer drugs are associated with toxicities of skin, hair, nails, and mucosa, accurate diagnosis and early recognition of potential reactions may reduce the significant morbidity, cosmetic disfigurement, and psychological distress.
ACKNOWLEDGMENT
Department of Oncology, Father Muller Medical College is acknowledged.
| 1. |
Susser WS, Whitaker-Worth DL, Grant-Kels JM. Mucocutaneous reactions to chemotherapy. J Am Acad Dermatol 1999;40:367-98.
[Google Scholar]
|
| 2. |
Joshi SS, Ortiz S, Witherspoon JN, Rademaker A, West DP, Anderson R, et al. Effects of epidermal growth factor receptor inhibitor-induced dermatologic toxicities on quality of life. Cancer 2010;116:3916-23.
[Google Scholar]
|
| 3. |
Kamil N, Kamil S, Ahmed SP, Ashraf R, Khurram M, Ali MO. Toxic effects of multiple anticancer drugs on skin. Pak J Pharm Sci 2010;23:7-14.
[Google Scholar]
|
| 4. |
Fabbrocini G, Cameli N, Romano MC, Mariano M, Panariello L, Bianca D, et al. Chemotherapy and skin reactions. J Exp Clin Cancer Res 2012;31:50.
[Google Scholar]
|
| 5. |
Balagula Y, Lacouture ME, Cotliar JA. Dermatologic toxicities of targeted anticancer therapies. J Support Oncol 2010;8:149-61.
[Google Scholar]
|
| 6. |
Heidary N, Naik H, Burgin S. Chemotherapeutic agents and the skin: An update. J Am Acad Dermatol 2008;58:545-70.
[Google Scholar]
|
| 7. |
Pragasam V, Verma R, Vasudevan B. Sorafenib and sunitinib: A dermatologist′s perspective. Indian Dermatol Online J 2014;5:1-3.
[Google Scholar]
|
| 8. |
Tsao AS, Kantarjian H, Cortes J, O′Brien S, Talpaz M. Imatinib mesylate causes hypopigmentation in the skin. Cancer 2003;98:2483-7.
[Google Scholar]
|
| 9. |
Arora B, Kumar L, Sharma A, Wadhwa J, Kochupillai V. Pigmentary changes in chronic myeloid leukemia patients treated with imatinib mesylate. Ann Oncol 2004;15:358-9.
[Google Scholar]
|
| 10. |
Baselga E, Drolet BA, Casper J, Esterly NB. Chemotherapy-associated supravenous hyperpigmentation. Dermatology 1996;192:384-5.
[Google Scholar]
|
| 11. |
Gropper CA, Don PC, Sadjadi MM. Nail and skin hyperpigmentation associated with hydroxyurea therapy for polycythemia vera. Int J Dermatol 1993;32:731-3.
[Google Scholar]
|
| 12. |
Cakir B, Sucak G, Haznedar R. Longitudinal pigmented nail bands during hydroxyurea therapy. Int J Dermatol 1997;36:236-7.
[Google Scholar]
|
| 13. |
Herrington JD, Figueroa JA. Severe necrosis due to paclitaxel extravasation. Pharmacotherapy 1997;17:163-5.
[Google Scholar]
|
| 14. |
Matos-Fernandez N, Ruiz Y, Rodriguez J, Baez L, Caceres W, Lopez-Pinto V, et al. Toxic epidermal necrolysis, an unusual adverse effect of Capecitabine. Cancer Ther 2008;6:271-4.
[Google Scholar]
|
| 15. |
Chen W, Yu YS, Liu YH, Sheen JM, Hsiao CC. Nail changes associated with chemotherapy in children. J Eur Acad Dermatol Venereol 2007;21:186-90.
[Google Scholar]
|
| 16. |
Hinds G, Thomas VD. Malignancy and cancer treatment-related hair and nail changes. Dermatol Clin 2008;26:59-68.
[Google Scholar]
|
| 17. |
James WD, Odom RB. Chemotherapy-induced transverse white lines in the fingernails. Arch Dermatol 1983;119:334-5.
[Google Scholar]
|
| 18. |
Yeager CE, Olsen EA. Treatment of chemotherapy-induced alopecia. Dermatol Ther 2011;24:432-42.
[Google Scholar]
|
| 19. |
Chiewchanvit S, Noppakun K, Kanchanarattanakorn K. Mucocutaneous complications of chemotherapy in 74 patients from Maharaj Nakorn Chiang Mai Hospital. J Med Assoc Thai 2004;87:508-14.
[Google Scholar]
|
| 20. |
Chadha V, Shenoi SD. Hair loss in cancer chemotherapeutic patients. Indian J Dermatol Venereol Leprol 2003;69:131-2.
[Google Scholar]
|
Fulltext Views
9,088
PDF downloads
3,307





