Translate this page into:
Dermoscope
Correspondence Address:
Uday Khopkar
Department of Dermatology, Seth GS Medical College & KEM Hospital, Parel, Mumbai - 400 012
India
| How to cite this article: Nischal K C, Khopkar U. Dermoscope. Indian J Dermatol Venereol Leprol 2005;71:300-303 |

 |
 |
 |
 |
 |
 |
 |
 |
A dermoscope (dermatoscope) is a non-invasive, diagnostic tool which visualizes subtle clinical patterns of skin lesions and subsurface skin structures not normally visible to the unaided eye. It has also been called a skin surface microscope, epiluminescence microscope or episcope. Some dermoscopic patterns are observed consistently with certain diseases and these then could be used for their diagnosis. Hence, this office procedure may obviate the need for a skin biopsy for diagnosis and for follow-up. The facility of storage of images and the results being immediately available are added advantages. Basically, a dermoscope is functionally similar to a magnifying lens but with the added features of an inbuilt illuminating system, a higher magnification which can be adjusted, the ability to assess structures as deep as in the reticular dermis, and the ability to record images.
Principle
The basic principle of dermoscopy is transillumination of a lesion and studying it with a high magnification to visualize subtle features.[1] Light incident on skin undergoes reflection, refraction, diffraction and absorption. These phenomena are influenced by physical properties of the skin [Figure - 1]. Most of the light incident on dry, scaly skin is reflected, but smooth, oily skin allows most of the light to pass through it, reaching the deeper dermis. This principle has been harnessed to improve the visibility of subsurface skin structures by employing application of linkage fluids over the lesions to be studied to improve the translucency of the skin. Various linkage fluids used are oils (immersion oil, olive oil and mineral oil), water, an antiseptic solution and glycerin. Immersion oil is not used because it contains chlorinated paraffin and dibutyl phthalate which have teratogenic, fetotoxic, and carcinogenic effects.[2] Water or antiseptic solutions evaporate quickly and hence are less preferred than oils. We have used liquid paraffin, which is inexpensive, safe and easily available, with good results. Glass has a refractive index (1.52) similar to that of skin (1.55) and hence when placed over oil-applied skin, further enhances transillumination of the lesion.
Basic design of a dermoscope
The essential components of a dermoscope are:
1. Achromatic lens: Most instruments provide 10x magnification, but higher magnifications can be achieved with special lenses.
2. Inbuilt illuminating system: Halogen lamps, which are oriented at an angle of 20[0], are placed within the handheld piece. The colour contrasts of lesions are altered by the yellow light of halogen lamp. Light emitting diodes (LED) (Delta 20Ó, DermLiteÓ) provide high intensity white light and consume 70% less power than halogen lamps. Illumination can be altered by turning off a set of LEDs. They are also designed to emit lights of different colors for better visualization of the skin as penetration of the skin by light is proportional to the wavelength of light (DermLite MSÓ).
3. Power supply: Handheld instruments are usually powered by batteries, e. g. lithium ion battery (DermLite DL100Ó, DermLite platinumÓ), rechargeable lithium battery (DermLite MSÓ, Dermlite Pro DP-RÓ), AA battery (mini 2000 dermatoscopeÓ), and rechargeable handles (DELTA 20Ó).
Additional facilities in some dermoscopes are an inbuilt photography system, either an attachable conventional or digital camera or an inbuilt camera, and supporting software, for the capture, storage, retrieval and even interpretation of images.
Types of dermoscopy instruments
Marghoob et al have exhaustively reviewed various models of dermoscopes.[4] For simplicity, dermoscopic instruments can be grouped as:
a) Instruments without image capturing facility, e.g. dermatoscope,
b) Instruments with image capturing facility, e.g. DermaphotÓ, and
c) Instruments with image capturing facility and analytical capability, e.g. DermoGenius MolemapÓ.
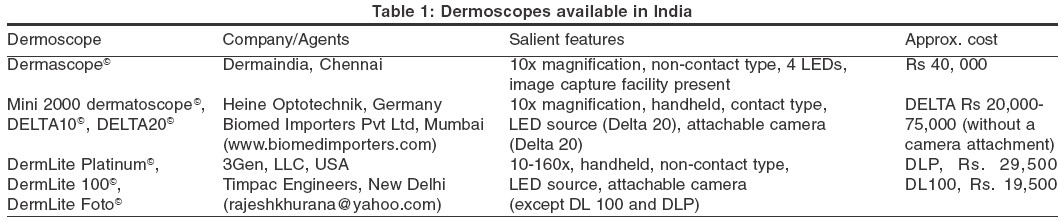
The latter two instruments have the added advantage of being able to take short videos of dermoscopy. Since digital cameras can now be fitted to some devices, this classification is not rigid. [Table - 1]
a) Dermoscopes without image capturing facility: A dermatoscope is a hand-held, otoscope-like instrument that lacks an inbuilt camera or any other image capture facility. However, cameras can be attached to some of these instruments with an adaptor, e.g. DermoGenius BasicÓ. Dermalite MSÓ (multispectral) incorporates four different colored polarized light, viz. white, blue (surface pigmentation), yellow (superficial vessels), and red (deep pigment and vessels), to facilitate better visualization of skin structures based on the principle that, depth of penetration of light is proportional to the wavelength.[5] Other models of this type are DELTA 10Ó, Mini 2000 DermatoscopeÓ, EpiscopeÓ.
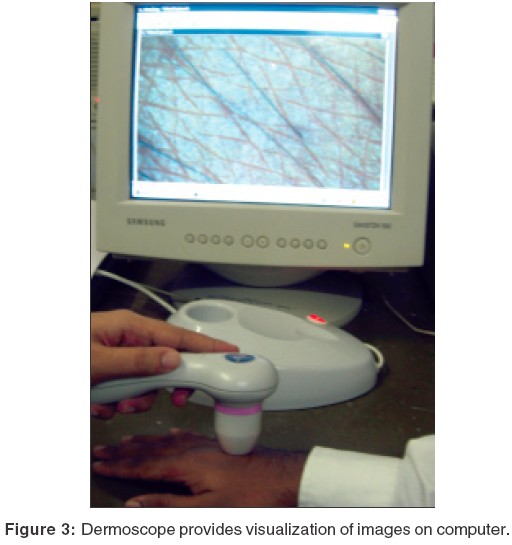
b) Dermoscopes with image capturing facility: These instruments have either an inbuilt image capture system or have a camera attached for dermoscopic photography. Also, whole body photography (body mapping) is possible with an apparatus like Mole Max I. Other instruments of this category are Delta 20Ó [Figure - 2], DermascopeÓ [Figure - 3], DermaphotÓ [Figure - 4], Dermlite FotoÓ and Video episcopeÓ. DermaphotÓ has a special lens, which can be mounted onto a conventional or a digital camera. Both clinical and dermoscopic pictures of 10x magnification can be taken. A videodermatoscope (Video dermascopeÓ, Videocap 100Ó) has a high resolution camera fitted to the hand piece. The image is seen on the computer screen. Small videos can be taken with this instrument.
c) Dermoscope with image capture facility and analytical capability: These instruments are mainly used in western countries where the concern of melanomas is a driving force to improve dermatoscopes for clinical diagnosis and preoperative assessment of pigmented lesions. Archived images of the patient can be compared with new ones and any significant change in lesion produces different colour signals. An artificial neural network mechanism helps judge whether a melanocytic nevus is benign, e.g. DermoGenius MoleMapÓ, Fotofinder dermoscopeÓ, Molemax IIÓ.
Technique
Dermoscopy can be done by either the non-contact or the contact technique. In the contact technique, the glass plate of the instrument comes in contact with the surface of the linkage fluid applied lesion. In contrast, in the non-contact technique, there is no contact of the lens with the skin; the cross-polarized lens absorbs all the scattered light and hence allows only light in a single plane to pass through it. While the non-contact technique ensures that there is no nosocomial infections,[3] this advantage is overshadowed by the disadvantages of decreased illumination and poor resolution.[4] Contact plates are made of multi-coated silicone glass and are of different types. Graduated plates have inscribed scales for measuring the lesion, while non-graduated plates lack a scale. Small plates have a small contact area to facilitate use in difficult to access regions like the web spaces, flexures and for nail fold capillaroscopy. Contact plates can be sterilized by using 2% glutaraldehyde, methylated spirit, boiling or autoclaving.
Uses
Dermoscopes have been largely used in white skinned individuals for the study of melanocytic nevi and melanoma. However, it can be used to diagnose other conditions too, e.g. psoriasis,[6] lichen planus,[6] dermatofibroma,[7] Darier′s disease,[8] cicatricial alopecia,[9] seborrheic keratosis[10] and urticarial vasculitis.[11] Dermoscopy has also been used to calculate the follicular density in the donor area before follicular unit hair transplantation[12] and in monitoring adverse effects of potent topical corticosteroids in psoriasis.[13]
Dermoscopes are not commonly used in developing countries because they are expensive and are not readily available. Their major use in the developed world is the study of melanocytic nevi and melanoma in white skinned individuals. Their potential for study of inflammatory and pigmentary dermatoses is waiting to be tapped.
| 1. |
William Stolz, Peter Bilek, Michael Landchaer, Amandcogneta. Basis of dermatoscopy and skin-surface microscopy. William Stolz, Peter Bilek, Michael Landchaer, Amandcogneta. Color atlas of dermatoscopy. 1st ed. Germany: Blackwell Publications; 1994.p.7-10.
[Google Scholar]
|
| 2. |
Binder M, Kittler H, Pehamberger H, Wolff K. Possible hazard to patients from immersion oil used for epiluminescence microscopy. J Am Acad Dermatol 1999; 40:499.
[Google Scholar]
|
| 3. |
Stauffer F, Kittler H, Forstinger C, Binder M. The dermatoscope: a potential source of nosocomial infection? Melanoma Res 2001;11:181.
[Google Scholar]
|
| 4. |
Marghoob AA, Swindle LD, Moricz CZM, Sanchez Negron FA, Slue B, Halpern AC, Kopf AW. Instruments and new technologies for the in vivo diagnosis of melanoma. J Am Acad Dermatol 2003;49;777-97.
[Google Scholar]
|
| 5. |
http://www.dermlite.com/dl2ms.html Last accessed on 30.07.2005.
[Google Scholar]
|
| 6. |
Vαzquez-L�pez F, Manjon-Haces JA, Maldonado-Seral C, Raya-Aguado C, Perez-Oliva N, Marghoob AA. Dermoscopic features of plaque psoriasis and lichen planus: new observations. Dermatology 2003;207:151-6.
[Google Scholar]
|
| 7. |
Ferrari A, Soyer H P, Peris K, Argenziano G, Mazzocchetti G, Piccolo D, et al. Central white scarlike patch: A dermatoscopic clue for the diagnosis of dermatofibroma. J Am Acad Dermatol 2000;43:1123-5.
[Google Scholar]
|
| 8. |
Vαzquez-L�pez F, Lopez-Escobar M, Maldonado-Seral C, Perez-Oliva N, Marghoob AA. The handheld dermoscope improves the recognition of giant pseudocomedones in Darier's disease. J Am Acad Dermatol 2004;50:454-55.
[Google Scholar]
|
| 9. |
Kossard S, Zagarella. Spotted cicatricial alopecia in dark skin. A dermoscopic clue to fibrous tracts. Australas J Dermatol 1993;34:49-51.
[Google Scholar]
|
| 10. |
Braun RP, Rabinovitz HS, Krischer J, Kreusch J, Oliviero M, Naldi L, et al. Dermoscopy of pigmented seborrheic keratosis: a morphological study. Arch Dermatol 2002;138:1556-60.
[Google Scholar]
|
| 11. |
Vαzquez-L�pez F, Maldonado-Seral C, Soler-Sαnchez T, Perez-Oliva N, Marghoob A A. Surface microscopy for discriminating between common urticaria and urticarial vasculitis. Rheumatology 2003;42:1079-82.
[Google Scholar]
|
| 12. |
http://www.hairtransplantnetwork.com/Hair_transplant news_articles/Follicular_Unit_Transplant_ Method_p3.asp Last accessed on 30.07.2005.
[Google Scholar]
|
| 13. |
Vαzquez-L�pez F, Marghoob AA. Dermoscopic assessment of long-term topical therapies with potent steroids in chronic psoriasis. J Am Acad Dermatol 2004;51:811-3.
[Google Scholar]
|
Fulltext Views
17,595
PDF downloads
4,279





