Translate this page into:
Dermoscopy and patch testing in patients with lichen planus pigmentosus on face: A cross-sectional observational study in fifty Indian patients
2 Department of Pathology, All India Institute of Medical Sciences, New Delhi, India
Correspondence Address:
Vinod Kumar Sharma
Department of Dermatology and Venereology, All India Institute of Medical Sciences, Ansari Nagar, New Delhi - 110 029
India
| How to cite this article: Sharma VK, Gupta V, Pahadiya P, Vedi KK, Arava S, Ramam M. Dermoscopy and patch testing in patients with lichen planus pigmentosus on face: A cross-sectional observational study in fifty Indian patients. Indian J Dermatol Venereol Leprol 2017;83:656-662 |
Abstract
Background: Lichen planus pigmentosus (LPP) is a common cause of facial melanosis in the dark-skinned population. At present, information on dermoscopy and patch testing in LPP is limited.Objectives: To describe dermoscopic findings and study the role of patch testing in patients with LPP on the face.
Methods: Facial lesions of 50 patients with LPP were studied dermoscopically, followed by histological evaluation. Patch and photopatch tests with the Indian Standard Series and Scandinavian series, respectively, and patient's own cosmetics were performed on all patients.
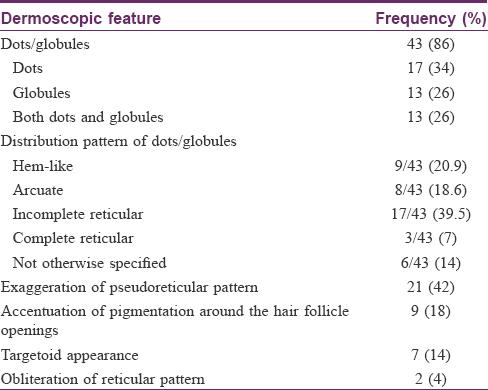
Results: The most common dermoscopic finding was dots and/or globules (43/50, 86%) in different patterns: hem-like (20.9%), arcuate (18.6%), incomplete reticular (39.5%), complete reticular (7%), and not otherwise specified (14%). Other patterns were exaggerated pseudoreticular pattern, accentuation of pigmentation around follicular openings, targetoid appearance, and obliteration of the pigmentary network. There were 26 relevant patch tests in 17 (34%) patients: para-phenylenediamine (n = 5), nickel (n = 3), colophony, perfume mix and fragrance mix (n = 2 each), thiuram mix and 3,3,4,5-tetrachlorosalicylanilide (n = 1 each), and patients' own products (n = 9). The only positive photopatch test was to fentichlor. No clinical or histological finding differed significantly based on patch test results. The only dermoscopic finding to be statistically associated with a positive patch test was the non-characteristic arrangement of dots/globules (P = 0.042).
Limitations: Dermoscopic features were not correlated with clinical features or disease duration. Implications of patch testing on the management of LPP cannot be commented upon as ours was a cross-sectional study.
Conclusions: The present study describes the dermoscopic findings of facial lesions in LPP. Our patch test results suggest a probable role of allergens in causing LPP on the face.
Introduction
Lichen planus pigmentosus (LPP) was first described by Bhutani et al., who considered it to be a macular variant of lichen planus.[1] It largely affects the dark-skinned population (Fitzpatrick skin phototypes IV and V), and is frequently encountered among Indians, Latin Americans, and Middle Easterners. Clinically, it is characterized by slate-gray-to-brown macules in a diffuse, blotchy, reticular, or perifollicular pattern affecting the sun-exposed and flexural sites. Histological features include varying degrees of pigment incontinence, basal cell damage, and band-like lymphohistiocytic infiltrate.[2],[3] The clinicopathological features of LPP are well-described. However, not much information is currently available regarding its dermoscopic and patch test findings. We undertook this study to describe the dermoscopic features in facial lesions of LPP and to explore the role of patch testing in these patients.
Methods
This was a prospective, observational study conducted in the department of dermatology and venereology, All India Institute of Medical Sciences, New Delhi, India over a period of 2 years (January 2013 to December 2014) after obtaining approval from the institutional ethics committee. Patients with a suspected diagnosis of LPP having facial lesions were screened for inclusion in the study. The diagnosis of LPP was based on the following previously described clinical and histopathological features: slate-gray-to-brown macules on the face with or without involvement of other sites (trunk, flexures) in the absence of preceding erythema or inflammation; histological evidence of pigment incontinence, with or without features of interface dermatitis (vacuolar basal cell degeneration, lichenoid infiltrate, necrotic keratinocytes, or colloid bodies) in the absence of epidermal spongiosis.[2],[3] Patients with other discernible causes of facial pigmentation viz. melasma, exogenous ochronosis, pigmentary demarcation lines, nevus of Ota, actinic lichen planus, post-inflammatory hyperpigmentation, and drug-induced hyperpigmentation were excluded based on history, examination and relevant investigations. After obtaining informed consent, clinical details of study participants were recorded. Dermoscopic examination of facial lesions was done by two dermatologists (VKS, VG) using a hand-held dermoscope (HEINE Delta mini ®, ×10 magnification). The digital images were captured using a mobile camera (Samsung GT-N700, 8 megapixels) with and without 4-fold magnification. The images were studied on the computer screen, and the following dermoscopic features were recorded after agreement between the two dermatologists: (1) exaggeration of the normal pseudoreticular pigmentary network, (2) obliteration of the normal pigmentary network, (3) brown dots and/or globules, (4) distribution pattern of dots and/or globules, and (5) accentuation of pigmentation around hair follicular openings. Apart from these findings, which were selected based on the available data and our personal experience, we also recorded any additional finding (s), if observed. A 3-mm punch biopsy was taken from the same site as that of dermoscopic examination. Hematoxylin and eosin stained sections were evaluated by two dermatopathologists (SA, MR) blinded to the dermoscopic findings. The following histological features were assessed: (1) degree of basal layer melanization, (2) pigment incontinence, (3) necrotic keratinocytes, (4) basal cell damage, (5) colloid bodies and (6) lichenoid inflammatory infiltrate. Closed patch test was performed on all patients using the Indian Standard Series (ISS). Patients' personal cosmetic products were also patch tested, if suspected. We did photopatch testing using the Scandinavian photopatch series on all study participants. Patch and photopatch tests were read after 48 and 96 hours, as per the International Contact Dermatitis Research Group guidelines.[4]
Statistical analysis
Clinical, dermoscopic, histological and patch test findings are presented as absolute numbers (percentages, %). Statistical association of dermoscopic features with relevant histological features and that of patch test results with clinical, histopathological, and dermoscopic findings was tested using Chi-square test and Fischer's exact test. P value of <0.05 was considered as statistically significant. Statistical analysis was performed using Stata 12 software (StataCorp. 2011. Stata Statistical Software: Release 12. College Station, TX: StataCorp LP).
Results
Fifty patients (11 males, 39 females) were enrolled in our study. All of them had dark complexion (Fitzpatrick skin type IV in 40 and V in 10 patients). The mean age was 35.86 ± 12.67 (range, 16–66) years, with 26 (52%) patients in the age-group of 20–40 years. Duration of pigmentation ranged from 5 months to 15 years (median 36 months). Forty one (82%) patients were using at least one cosmetic product before the onset of pigmentation, of whom 19 (46.3%) were using more than one cosmetic. Twenty nine patients were users of hair color products (hair dye or henna), twenty six used skin fairness or anti-ageing creams, twelve used moisturizers, and eight used fragrances. None gave history of itching or erythema preceding the pigmentation. Apart from the face, extra-facial sites were also affected in 24 (48%) patients. The most common colour of the facial pigmentation was slate-gray, while the commonest pattern was diffuse pigmentation [Figure - 1]. Two patients had coexistent classical oral lichen planus. [Table - 1] shows the clinical details of the study participants.
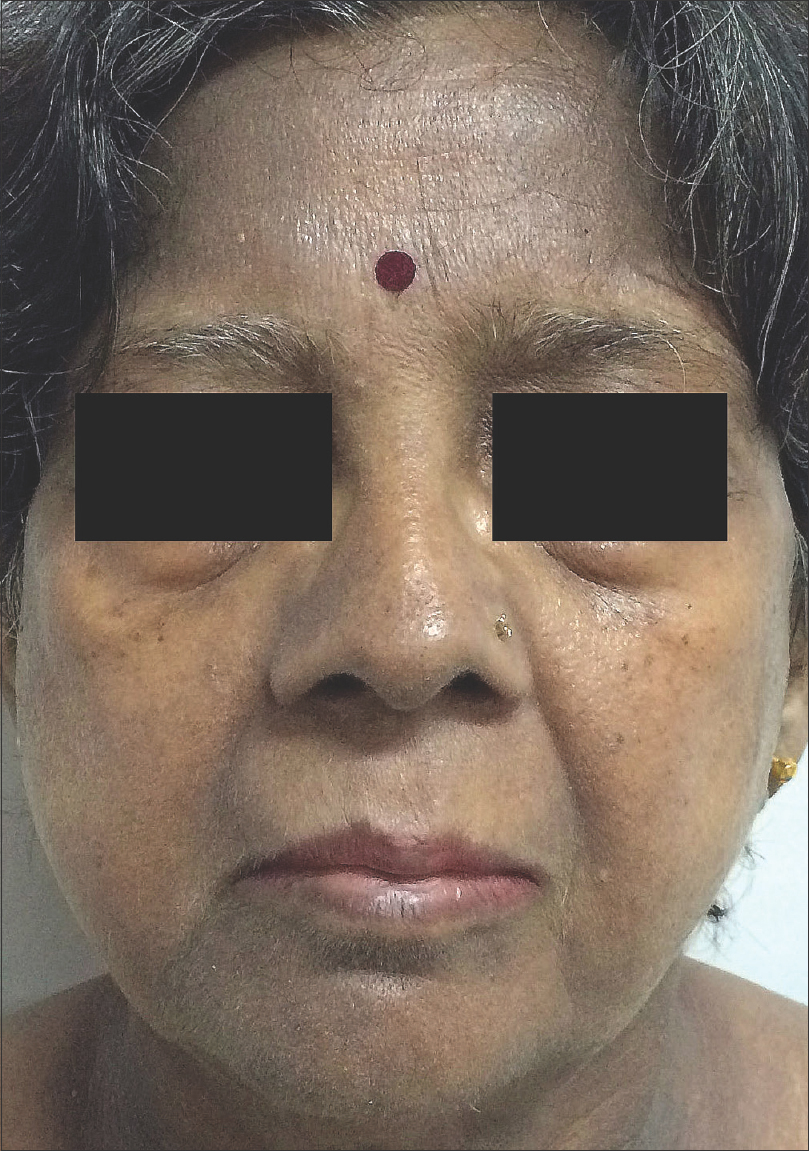 |
| Figure 1: Diffuse slate-gray pigmentation, most prominent on the forehead, nose, cheeks, pre-auricular and peri-oral area of a 49-year-old woman |

Dermoscopic features
Dermoscopic findings are summarized in [Table - 2]. The most common dermoscopic finding was the presence of dots and/or globules, seen in 43 (86%) cases (dots, n = 17; globules, n = 13; and both dots and globules, n = 13). The dots and globules were distributed in different patterns: (1) hem-like (interrupted linear) [Figure - 2]: 9/43 (20.9%); (2) arcuate (arciform): 8/43, (18.6%); (3) incomplete reticular (forming incomplete nets) 17/43 (39.5%); (4) complete reticular (forming complete nets) [Figure - 3]: 3/43 (7%); and (5) not otherwise specified (no specific pattern): 6/43 (14%). The next common dermoscopic finding was exaggerated pseudoreticular network seen in 21 (42%) cases. Dots and globules were accompanied by exaggerated pseudoreticular pigmentary network in 17 cases [Figure - 4]. Accentuation of pigmentation around hair follicle openings was seen in 9 (18%) cases [Figure - 5], whereas obliteration of the pigmentary network by pigment deposition was seen in 2 (4%) cases. “Targetoid” appearance (a central brown dot surrounded by hypopigmented halo), a finding not initially chosen for dermoscopic evaluation, was seen in 7 cases [Figure - 6].
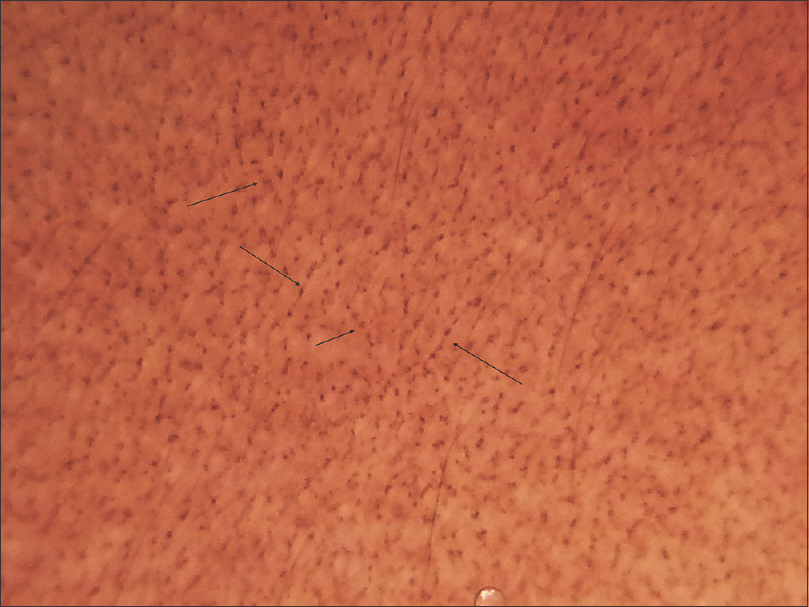 |
| Figure 2: Dermoscopy shows dots and globules in a ‘hem-like’ pattern (black arrows) |
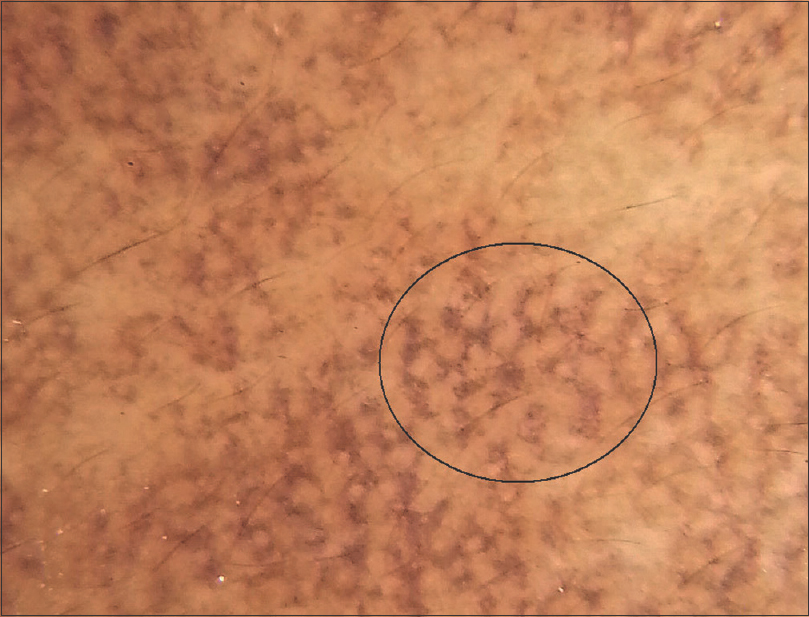 |
| Figure 3: Dermoscopy shows patchy exaggeration of pseudoreticular network and dots /globules in a ‘complete reticular‘ pattern (black circle) |
 |
| Figure 4: Dermoscopy shows dots and globules (black circles), and exaggerated pseudoreticular network (black squares), with sparing of follicular openings (yellow squares) |
 |
| Figure 5: Dermoscopy showing accentuation of pigment around the follicular openings |
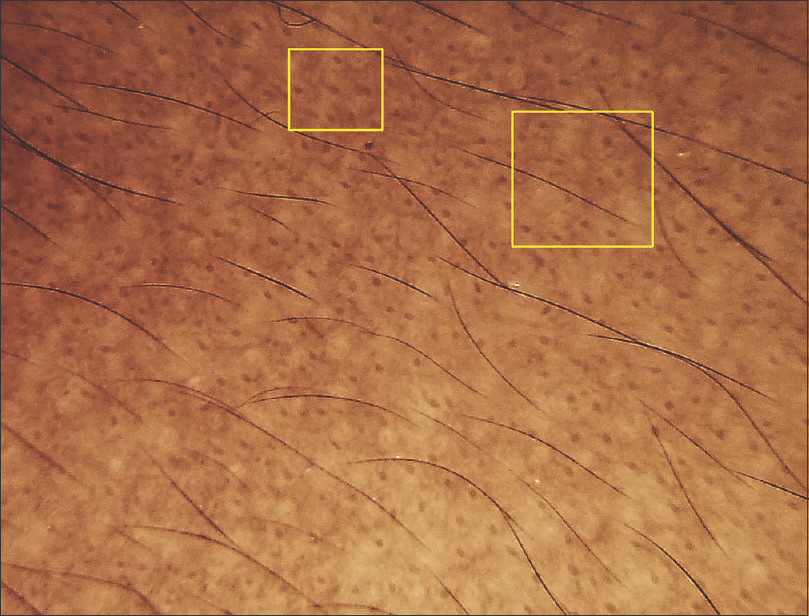 |
| Figure 6: Dermoscopy shows exaggerated pseudoreticular pattern and targetoid appearance (central dot with surrounding halo, yellow squares) |
Histopathological features
Presence of melanophages in upper dermis was observed in all the biopsy specimens. Pigment incontinence was categorized as mild (less than 10 melanophages/×400 field), moderate (10–20 melanophages/×400 field), and severe (more than 20 melanophages/×400 field) seen in 42% (n = 21/50), 24% (n = 12/50), and 34% (n = 17/50) biopsy samples, respectively [Figure - 7]. Prominent basal layer melanization (n = 29, 58%), necrotic keratinocytes (n = 10, 20%), colloid bodies (n = 9, 18%), basal cell vacuolisation (epidermal in 15, 30%; follicular in 4, 8%), and upper dermal band-like lymphocytic infiltrate (n = 4, 8%) were the other histological findings. Follicular plugs were noted in 9 (18%) cases.
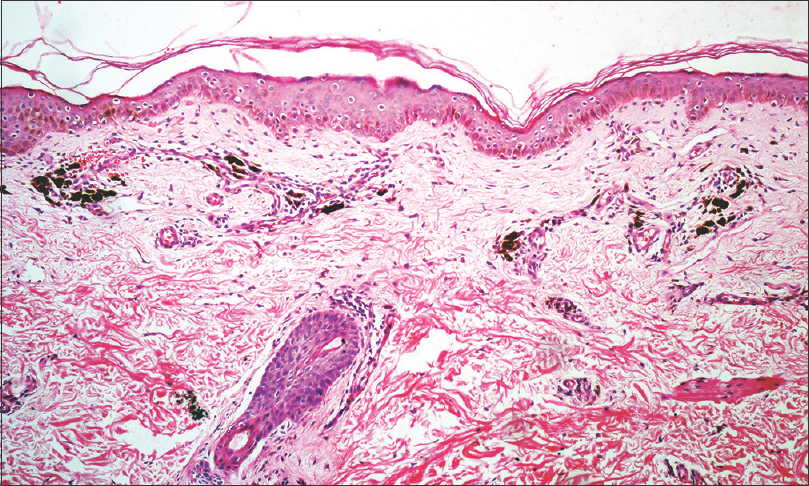 |
| Figure 7: Histopathology shows an unremarkable epidermis, moderate pigment incontinence and mild perivascular lympho-histiocytic infiltrate in upper dermis (H & E, 100X) |
Dermoscopic findings of dots with/without globules was significantly associated with moderate-severe pigment incontinence histologically (P = 0.021). Exaggeration of the pseudoreticular pigmentary network did not correlate well with prominent basilar melanisation (P = 0.917) or pigment incontinence (P = 0.390), while targetoid appearance on dermoscopy showed a significant association with follicular plugging on histology (P< 0.001).
Patch and photopatch test results
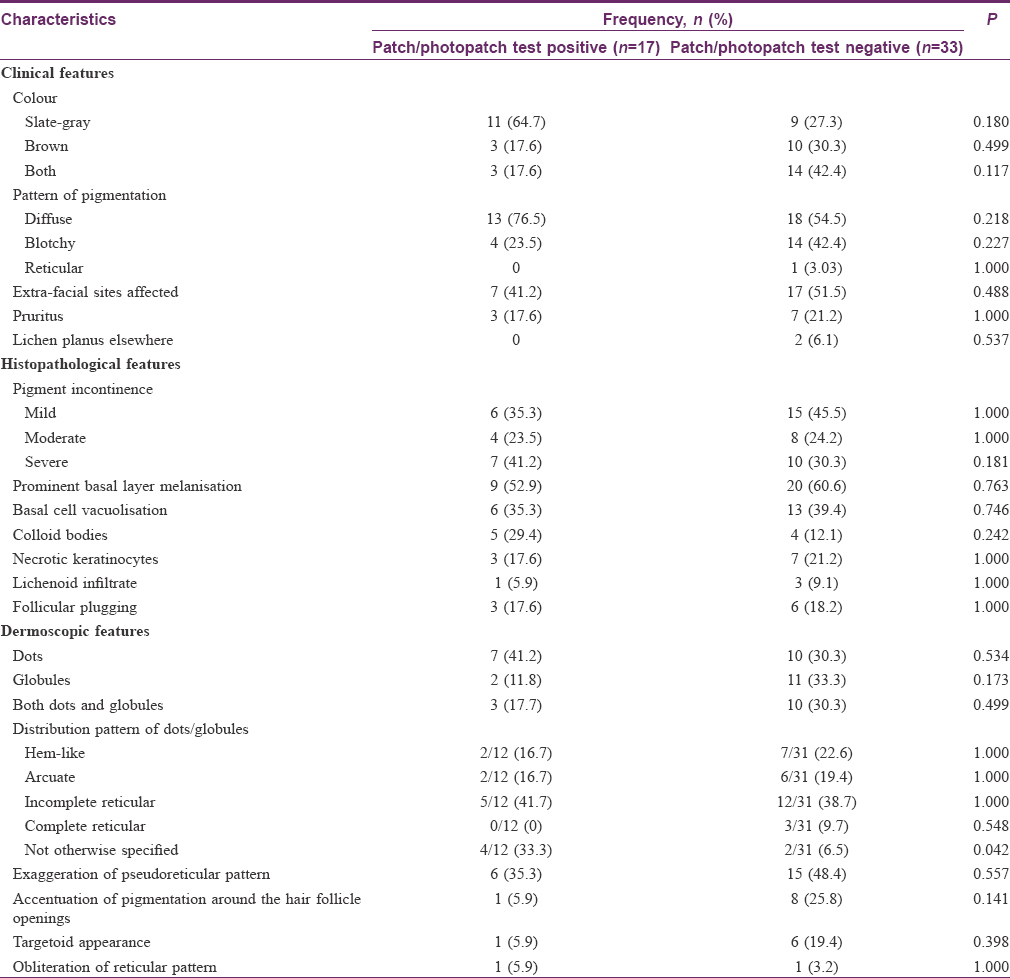
A total of 28 patch tests were positive, of which 26 (92.8%) were relevant, in 17/50 (34%) patients. Sixteen allergens were positive, of which para-phenylenediamine (PPD) was the most common (n = 5; 4 were ++, 1 was +), followed by nickel sulphate (n = 3; 2 were ++, 1 was +), colophony (n = 2; ++ and +), perfume mix (n = 2; both +) and fragrance mix (n = 2; both +), thiuram mix, 3, 3, 4, 5-tetrachlorosalicylanilide 0.1%, nitrofurantoin and neomycin sulphate (n = 1 each; all +), and personal cosmetic products (commercial hair dyes, n = 4; three were ++ and 1 was +, shaving cream, n = 3; all +, skin-fairness cream and moisturizer, n = 1 each, both +). The only photopatch test positive was to fentichlor 0.1% (+). To establish the relevance of positive results to the current facial pigmentation, a careful re-evaluation of patients' history, including exposure to cosmetics containing these allergens prior to the onset of pigmentation, and pattern of pigmentation was done. Positive patch tests to all allergens except nitrofurantoin and neomycin sulphate were considered relevant. Seven patients reacted to more than one allergen. All four patients with a positive patch test to commercial hair dyes also tested positive to PPD. Three of these patients had an additional positive patch test to nickel sulphate, colophony, and neomycin sulphate. One patient had a positive patch test to PPD and moisturizing cream. Two patients tested positive to perfume mix and fragrance mix, one of whom had a positive patch test with colophony as well.
The clinical, histopathological, and dermoscopic findings based on patch test results are summarized in [Table - 3]. There were no statistically significant differences in the clinical features or histological findings between patients with positive and negative patch tests. Apart from the non-characteristic arrangement of dots/globules, which was statistically associated with a positive patch test result (P = 0.042), no other dermoscopic finding was found to be statistically significantly different between these two groups.
Discussion
In the present study, we have described the spectrum of dermoscopic findings in facial lesions of LPP and identified their possible histopathological correlates. In addition, patch and photopatch testing was performed to study the role of contact allergens in causing facial pigmentation in such patients, and an attempt was made to find if any clinical, histopathologic, or dermoscopic findings are different in patients with a positive patch test. Dots and/or globules were the most common dermoscopic findings in our study and correlated well with significant pigment incontinence on histology. This is consistent with the current understanding.[5] We also noted various distribution patterns of dots and globules like “hem-like,” “arcuate,” “incomplete reticular,” and “complete reticular,” resembling different stages of the pigmentary network of skin. It is possible that these different patterns represent varying degrees of pigment incontinence, i.e., mild cases show hem-like and arcuate pattern whereas more severe pigmentation demonstrates incomplete and complete reticular network. Further work is required to correlate these dermoscopic patterns with clinical and histological findings. The finding of a central dot surrounded by a hypopigmented halo, resembling a targetoid lesion, corresponded with follicular plugging on histopathology. Because keratotic follicular plugs can be seen commonly on the face, this finding may not necessarily be a clue to the diagnosis of LPP, and has been described in other unrelated diseases on the face as well.[6],[7],[8]
Information regarding the dermoscopic features of LPP is gradually emerging. Gray-brown granular dots were reported in a case of axillary lichen planus pigmentosus inversus.[9] Dots and globules have also been described in some patients with LPP, as a part of a larger series of lichen planus variants.[10] Recently, Pirmez et al. described dermoscopic features of facial LPP in 37 patients, who also had coexistent frontal fibrosing alopecia.[11] The various observed patterns included pseudonetwork and dots (dotted/speckled blue-gray dots/blue-gray dots in circles), which are quite similar to our findings. Their finding of blue-gray dots in circles probably corresponds to our finding of accentuation of pigmentation around follicle openings. Other findings included vascular alterations and loss of facial vellus hair; these were not noted among our patients. Though the authors speculated that dermoscopic pigmentation patterns are due to dermal melanophages resulting from interface dermatitis, a correlation analysis with histopathological features was not undertaken. Dermoscopy may prove beneficial in differentiating among the various heterogeneous entities which can present as facial melanosis, such as LPP, pigmented cosmetic dermatitis, ashy dermatosis, melasma, exogenous ochronosis, maturational dyschromia, and facial acanthosis nigricans; however, its utility in such a setting has not been formally evaluated.[11],[12],[13],[14] Approximately one-third of our LPP patients had a positive patch test. Tienthavorn et al. also obtained a similar figure (4 out of 11, 36.36%) in Thai patients with LPP who were patch tested with cosmetic and fragrance series, irrespective of the facial involvement.[15] Para-phenylenediamine and commercial hair dyes were the most common allergens in our study, and they have been reported to produce positive patch test results in LPP patients previously as well.[16] Nickel is present in many facial cosmetics as a contaminant, especially eye cosmetics, and was the second most common allergen in our series.[17] It has been implicated as an important allergen in cosmetic dermatitis.[18] Tienthavorn et al. found nickel to be the most common allergen in their study of 43 patients with lichen planus pigmentosus, pigmented contact dermatitis, and erythema dyschromicum perstans.[15] Apart from the usual allergens with cosmetic sensitivity, we also found positive patch tests to thiuram mix and 3, 3, 4, 5-tetrachlorosalicylanilide. Rubber cosmetic applicators such as eyeshadow sponge and eyelash curlers contain thiuram mix, whereas 3, 3, 4, 5-tetrachlorosalicylanilide is found in soaps, shampoos, deodorants, and in some textiles. Photopatch testing was positive only to fentichlor, a topical antifungal and antibacterial found in cosmetics and a known photoallergen. These results are in contrast to the pattern of contact sensitivity generally observed in contact dermatitis clinics in India, where nickel and potassium dichromate are the most common allergens followed by neomycin, mercaptobenzothiazole, fragrance mix, and cobalt chloride.[19],[20] The high prevalence of contact sensitivity to PPD/hair dyes in our study is, in fact, similar to what has been observed in Indian patients with suspected cosmetic dermatitis.[21] Whether the facial melanosis in our patients having a positive patch test represents pigmented cosmetic dermatitis instead of LPP is debatable. It is possible that cosmetics play a role in causing LPP on the face, as suggested by positive patch tests with PPD, hair dyes, and other cosmetics.[16],[22] Because of several overlapping clinical and histological features, distinguishing pigmented cosmetic dermatitis from LPP can be very difficult. Some workers do not consider them as distinct entities but rather a spectral manifestation of the same disease process.[16] We did not find any clinical feature to be a clue to a positive patch test result. Moreover, except for the non-characteristic pattern of dots/globules, which was significantly more common in patients with a positive patch test, the dermoscopic findings were also comparable. This was expected as there were no significant differences in the histological features between the two groups. In the absence of major differences in clinical, histopathological, or dermoscopic features, whether to label such pigmentary dermatoses as LPP or pigmented cosmetic dermatitis may be a question of semantics only. However, it would be interesting to note if avoidance of implicated contact allergens can lead to an improvement in pigmentation.
Our study has certain limitations. We did not correlate the dermoscopic findings with clinical features or disease duration. Using a cosmetic series for patch testing could have identified additional contact allergens. Finally, the implications of patch testing on the management of LPP cannot be commented upon by this cross-sectional observational study, and future longitudinal studies are required to address this question.
Conclusion
We have described the spectrum of clinical, histological, and dermoscopic features of facial lesions of LPP, and have correlated the dermoscopic findings with those on histology. Most common dermoscopic findings were dots and globules, which correlated well with histological evidence of pigment incontinence. Exaggerated pseudoreticular pattern and accentuation of pigmentation around hair follicle openings were other common findings. Though preliminary, these findings may lay the basis for distinguishing LPP from dermatoses which resemble it clinically and histologically. Our patch test results suggest a probable role of allergens/cosmetics in causing LPP on face.
Acknowledgment
We would like to acknowledge the assistance of Ms. Mona Pathak, Department of Biostatistics, All India Institute of Medical Sciences, New Delhi, India for her help in statistical analysis.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patients have given their consent for their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Bhutani LK, Bedi TR, Pandhi RK, Nayak NC. Lichen planus pigmentosus. Dermatologica 1974;149:43-50.
[Google Scholar]
|
| 2. |
Kanwar AJ, Dogra S, Handa S, Parsad D, Radotra BD. A study of 124 Indian patients with lichen planus pigmentosus. Clin Exp Dermatol 2003;28:481-5.
[Google Scholar]
|
| 3. |
Al-Mutairi N, El-Khalawany M. Clinicopathological characteristics of lichen planus pigmentosus and its response to tacrolimus ointment: An open label, non-randomized, prospective study. J Eur Acad Dermatol Venereol 2010;24:535-40.
[Google Scholar]
|
| 4. |
Wilkinson DS, Fregert S, Magnusson B, Bandmann HJ, Calnan CD, Cronin E, et al. Terminology of contact dermatitis. Acta Derm Venereol 1970;50:287-92.
[Google Scholar]
|
| 5. |
Braun RP, Scope A, Marghoob AA, Kerl K, Rabinovitz HS, Malvehy J. Histopathologic tissue correlations of dermoscopic structures. In: Atlas of Dermoscopy. 2nd ed. Ed. Marghoob AA, Malvehy J, Braun RP. London: Informa Healthcare; 2012. pp10-32.
[Google Scholar]
|
| 6. |
Zalaudek I, Giacomel J, Schmid K, Bondino S, Rosendahl C, Cavicchini S, et al. Dermatoscopy of facial actinic keratosis, intraepidermal carcinoma, and invasive squamous cell carcinoma: A progression model. J Am Acad Dermatol 2012;66:589-97.
[Google Scholar]
|
| 7. |
Zalaudek I, Giacomel J, Argenziano G, Hofmann-Wellenhof R, Micantonio T, Di Stefani A, et al. Dermoscopy of facial nonpigmented actinic keratosis. Br J Dermatol 2006;155:951-6.
[Google Scholar]
|
| 8. |
Lallas A, Argenziano G, Apalla Z, Gourhant JY, Zaballos P, Di Lernia V, et al. Dermoscopic patterns of common facial inflammatory skin diseases. J Eur Acad Dermatol Venereol 2014;28:609-14.
[Google Scholar]
|
| 9. |
Murzaku EC, Bronsnick T, Rao BK. Axillary lichen planus pigmentosus-inversus: Dermoscopic clues of a rare entity. Diagnosis: Lichen planus pigmentosus (LPP). J Am Acad Dermatol 2014;71:e119-20.
[Google Scholar]
|
| 10. |
Güngör S, Topal IO, Göncü EK. Dermoscopic patterns in active and regressive lichen planus and lichen planus variants: A morphological study. Dermatol Pract Concept 2015;5:45-53.
[Google Scholar]
|
| 11. |
Pirmez R, Duque-Estrada B, Donati A, Campos-do-Carmo G, Valente NS, Romiti R, et al. Clinical and dermoscopic features of lichen planus pigmentosus in 37 patients with frontal fibrosing alopecia. Br J Dermatol 2016;175:1387-90.
[Google Scholar]
|
| 12. |
Khunger N, Kandhari R. Dermoscopic criteria for differentiating exogenous ochronosis from melasma. Indian J Dermatol Venereol Leprol 2013;79:819-21.
[Google Scholar]
|
| 13. |
Gil I, Segura S, Martínez-Escala E, Lloreta J, Puig S, Vélez M, et al. Dermoscopic and reflectance confocal microscopic features of exogenous ochronosis. Arch Dermatol 2010;146:1021-5.
[Google Scholar]
|
| 14. |
Wang L, Xu AE. Four views of Riehl's melanosis: Clinical appearance, dermoscopy, confocal microscopy and histopathology. J Eur Acad Dermatol Venereol 2014;28:1199-206.
[Google Scholar]
|
| 15. |
Tienthavorn T, Tresukosol P, Sudtikoonaseth P. Patch testing and histopathology in Thai patients with hyperpigmentation due to erythema dyschromicum perstans, lichen planus pigmentosus, and pigmented contact dermatitis. Asian Pac J Allergy Immunol 2014;32:185-92.
[Google Scholar]
|
| 16. |
Bhutani LK. Pigmented contact dermatitis vs. lichen planus pigmentosus. Int J Dermatol 1977;16:860-2.
[Google Scholar]
|
| 17. |
Hepp NM, Mindak WR, Gasper JW, Thompson CB, Barrows JN. Survey of cosmetics for arsenic, cadmium, chromium, cobalt, lead, mercury, and nickel content. J Cosmet Sci 2014;65:125-45.
[Google Scholar]
|
| 18. |
Trattner A, Hodak E, David M. Screening patch tests for pigmented contact dermatitis in Israel. Contact Dermatitis 1999;40:155-7.
[Google Scholar]
|
| 19. |
Bajaj AK, Saraswat A, Mukhija G, Rastogi S, Yadav S. Patch testing experience with 1000 patients. Indian J Dermatol Venereol Leprol 2007;73:313-8.
[Google Scholar]
|
| 20. |
Handa S, Jindal R. Patch test results from a contact dermatitis clinic in North India. Indian J Dermatol Venereol Leprol 2011;77:194-6.
[Google Scholar]
|
| 21. |
Mehta SS, Reddy BS. Pattern of cosmetic sensitivity in Indian patients. Contact Dermatitis 2001;45:292-3.
[Google Scholar]
|
| 22. |
Nakayama H, Harada R, Toda M. Pigmented cosmetic dermatitis. Int J Dermatol 1976;15:673-5.
[Google Scholar]
|
Fulltext Views
12,006
PDF downloads
2,135





