Translate this page into:
Destructive facial plaque with massive cervical lymphadenopathy
Corresponding author: Dr. Keshavmurthy Vinay, Department of Dermatology, Venereology and Leprology, Postgraduate Institute of Medical Education & Research, Chandigarh, India. vinay.keshavmurthy@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Bakshi S, Sharma K, Vinay K, Chatterjee D, Dogra S. Destructive facial plaque with massive cervical lymphadenopathy. Indian J Dermatol Venereol Leprol 2023;89:609–12.
A 32-years-old female with no known comorbidities presented with bilateral cervical lymphadenopathy and an erythematous plaque over the right side of face of three years duration. The patient did not recall any history of trauma prior to the onset of lesions. She had received anti-tubercular therapy twice in the past for six months each for suspected tuberculosis (based on cytology features) with little improvement. General examination revealed bilateral firm, non-tender, matted submandibular and cervical lymphadenopathy, the largest measuring 5×3 cm in size. Cutaneous examination revealed single well to ill-defined plaque with few satellite lesions involving the right side of the face and scalp with overlying erosions and yellowish crusting [Figure 1]. Computed tomography scan revealed multiple enlarged bilateral lymph nodes in neck at levels I-VI with homogeneous enhancement along with diffuse skin and subcutaneous tissue edema on the right side of face. Biopsies were obtained from the facial plaque [Figure 2A] and lymph node [Figure 2B].
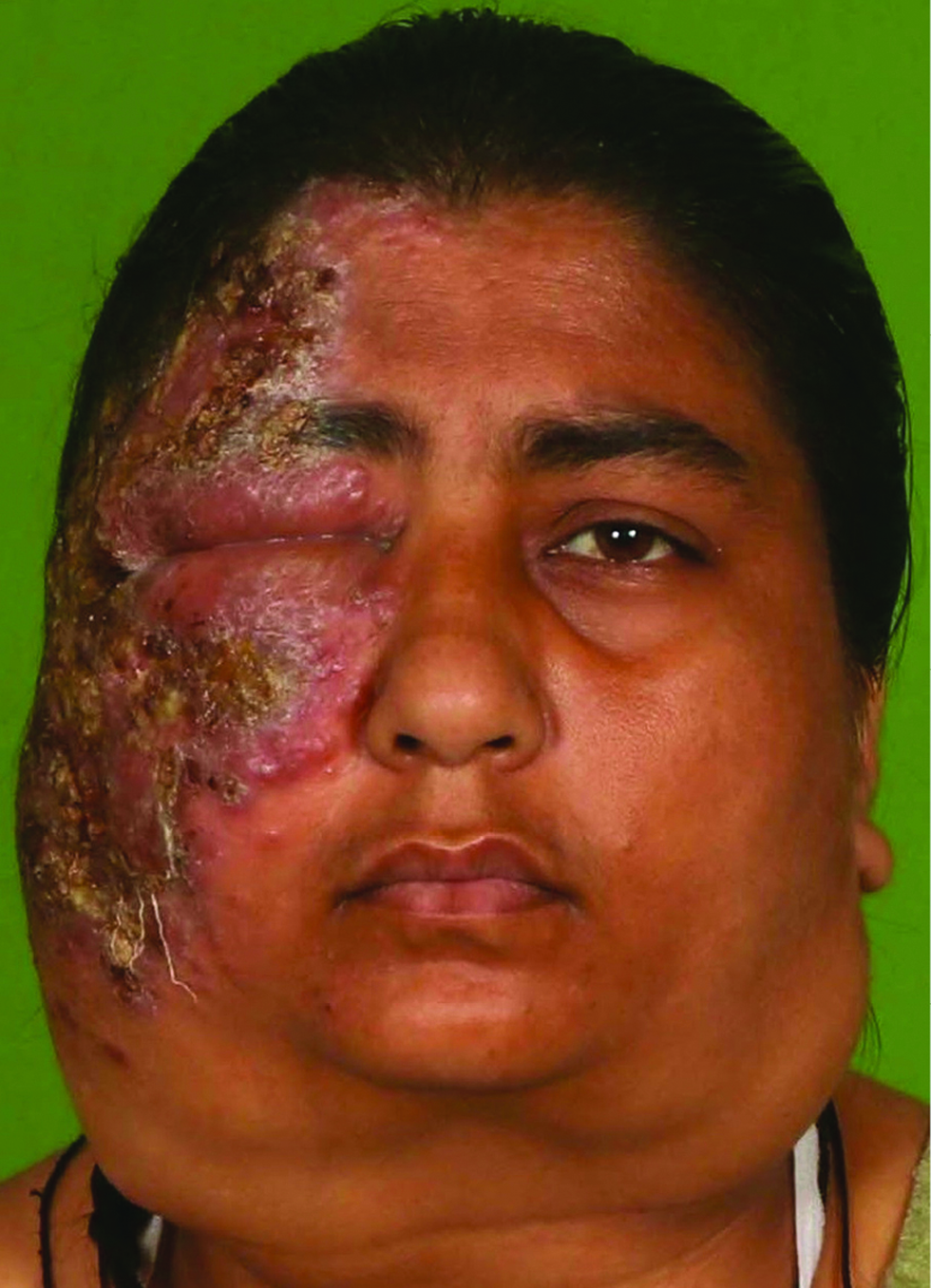
- A 32-years-old female showing bilateral massive lymphadenopathy. An erythematous well to ill-defined plaque involving the right side of the face, extending to scalp, forehead, eyelids, cheeks and upper part of the neck with overlying erosions and yellowish crusting can be appreciated
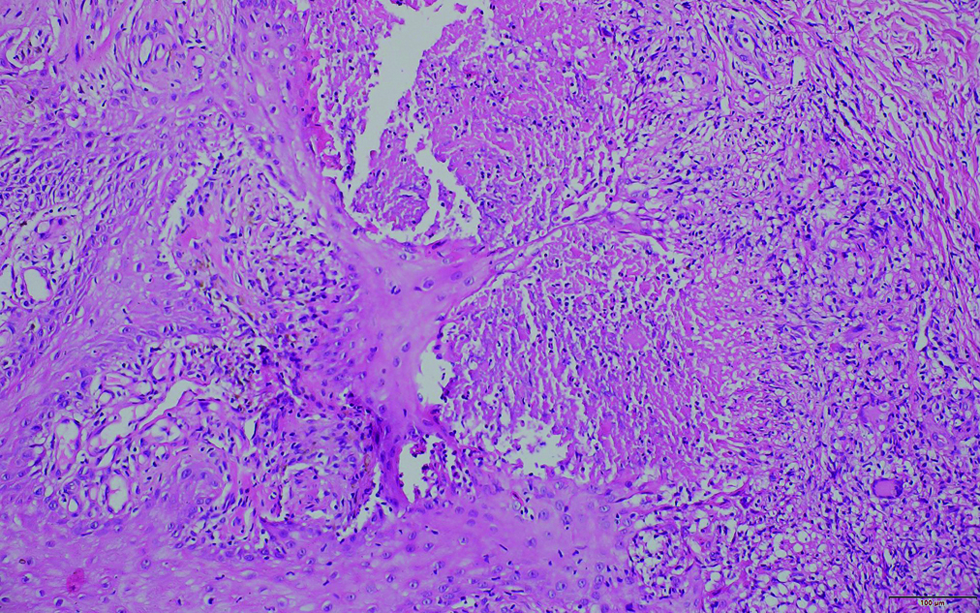
- Histopathology of section of skin shows multiple foci of necrotizing granulomas containing epithelioid histiocytes, Langhan's giant cells, and lymphocytic infiltrate in the dermis (H & E ×200)
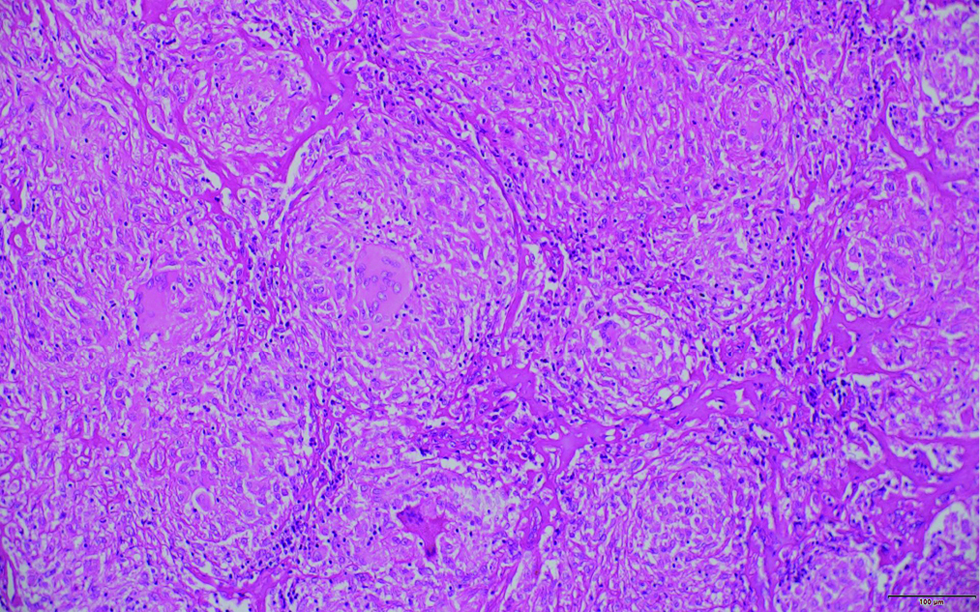
- Histopathology of section of lymph node showing numerous caseating epithelioid cell granulomas, causing its complete architectural effacement (H & E, ×200)
What is Your Diagnosis?
Diagnosis
Nontuberculous mycobacterial infection
Microscopic findings and laboratory investigation
On histopathological examination of the skin lesions and cervical lymph node, granulomatous inflammation with central caseous necrosis was seen. Acid-fast bacilli were observed on lymph node biopsy [Figure 3]. Mycobacterial cultures failed to grow the relevant organisms. Mycobacterium avium was identified in the lymph nodal tissue by polymerase chain reaction. On further investigations there was no evidence of an immunocompromised state; HIV serology was negative, T cell subsets were within normal range along with normal expression of interferon gamma receptor 1. Following 16 weeks of treatment with rifampicin 600 mg, clarithromycin 500 mg and minocycline 100 mg OD, there was significant improvement [Figure 4]. No adverse effects were observed.
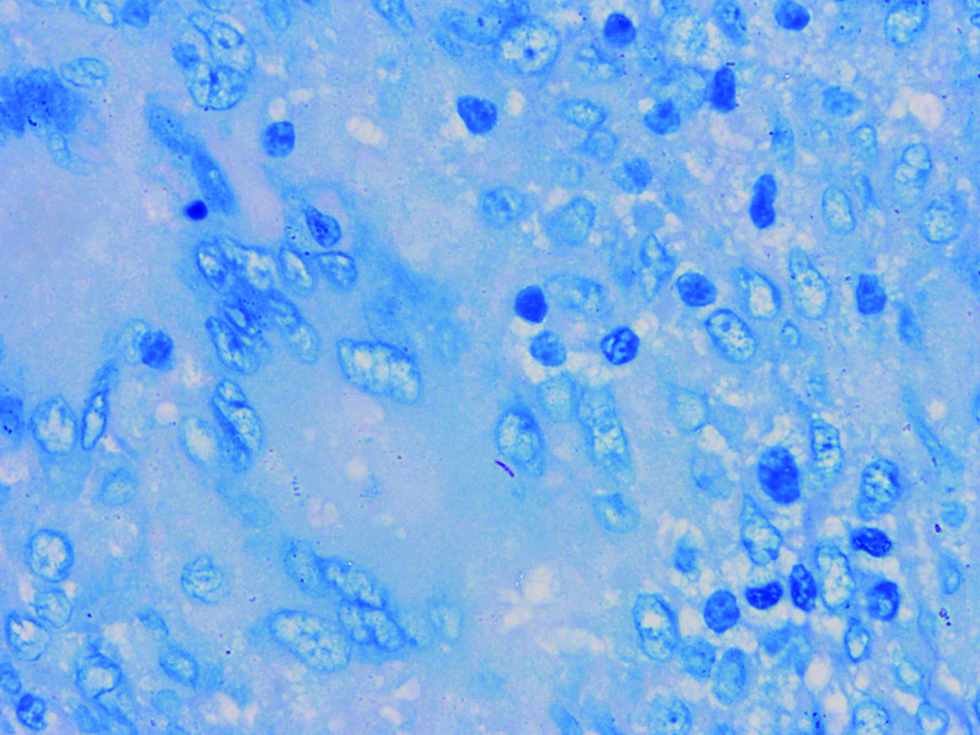
- Section of lymph node showing acid-fast bacilli (Ziehl-Neelsen stain ×1000)
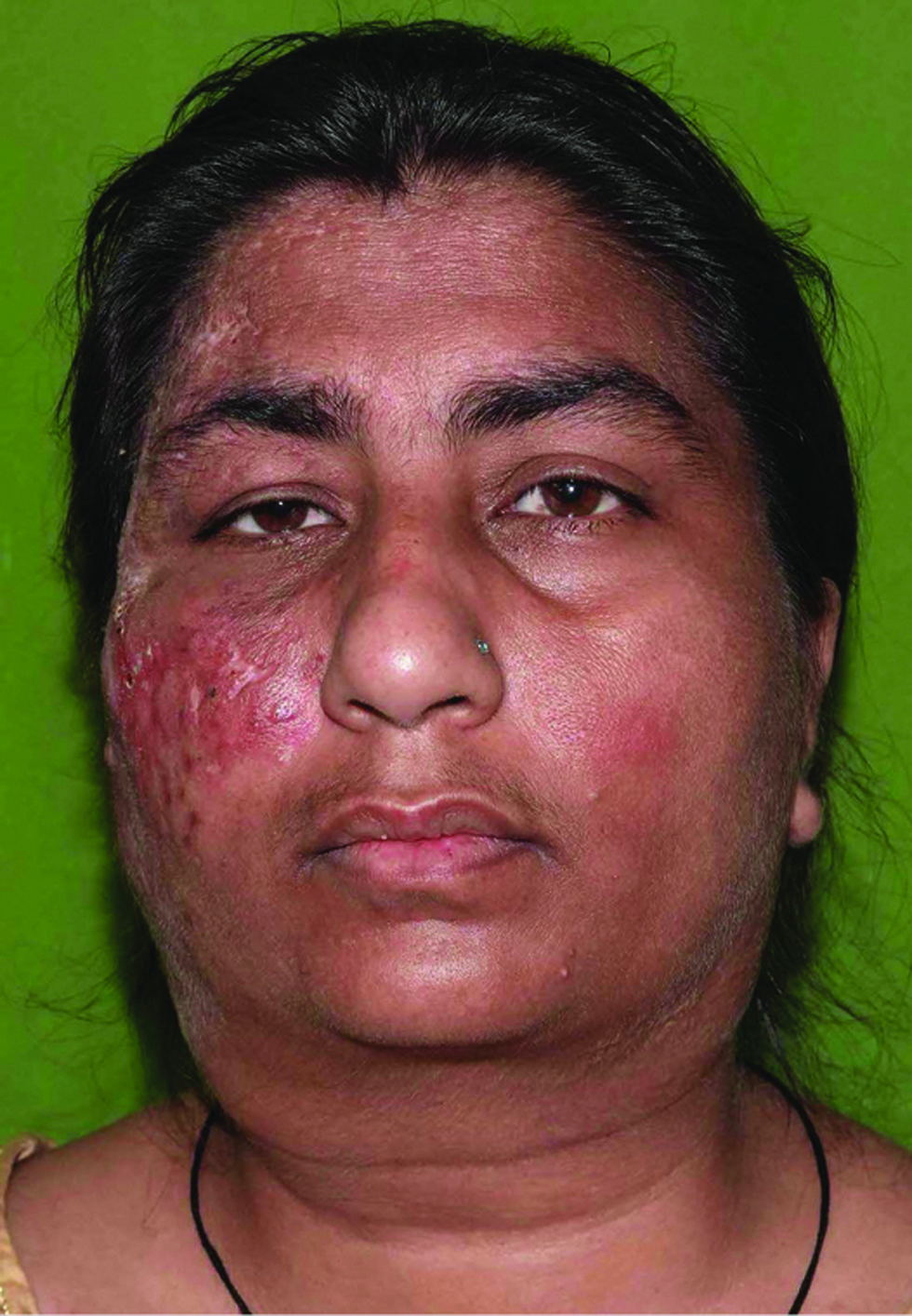
- Resolution of lymphadenopathy and healing of the facial plaque with scarring after treatment with rifampicin, clarithromycin and minocycline for 16 weeks
Discussion
Non-tuberculous mycobacteria also known as atypical mycobacteria or mycobacteria other than tuberculosis include mycobacterial pathogens other than Mycobacterium tuberculosis complex and Mycobacterium leprae. These include over 150 different species that are classified into rapidly growing and slowly growing mycobacteria.1
Mycobacterium avium complex encompasses many subspecies of slow-growing mycobacteria including Mycobacterium avium, Mycobacterium intracellulare and Mycobacterium chimaera. They are ubiquitous in the environment and are commonly found in soil and water sources. Mycobacterium avium complex infection is typically associated with pulmonary disease in an immunocompromised host, but there has been an increasing incidence of both pulmonary and extra-pulmonary infections (including skin and soft tissue infections, musculoskeletal infection, lymphadenitis and disseminated disease) in immunocompetent individuals.2
Skin and soft tissue infections caused by nontuberculous mycobacteria classically develop after traumatic injury, cosmetic procedures, or surgery, which can expose wounds to the soil, water, or medical devices contaminated with environmental mycobacteria. Cutaneous infections caused by the Mycobacterium avium complex can take the form of non-specific lesions including cellulitis, abscesses, folliculitis, pustules, ulcers, ecthyma-like lesions, nodules, infiltrated erythematous plaques, scaling plaques, verrucous ulcers, draining sinuses, sporotrichosis-like lesions, and panniculitis.
The clinical differentials of mycobacterial infection, lupoid leishmaniasis, deep fungal infection, Rosai-Dorfman disease, ulcerative sarcoidosis and Kikuchi disease were considered in our case which is further elaborated in Table 1. Diagnosis of non-tuberculous mycobacteria may be missed due to a lack of clinical suspicion and the difficulty in identifying the organisms by culture owing to very specific growth requirements in solid or liquid culture media. In such scenarios, a polymerase chain reaction may play an important role in identifying the organism as well as the species. However, the unavailability of laboratory facilities in resource-limited settings often leads to the treatment of these cases as drug-resistant tuberculosis.
|
Atypical mycobacterial infection |
Deep fungal infection |
Lupoid leishmaniasis |
Rosai-Dorfman disease |
Kikuchi disease |
|
|---|---|---|---|---|---|
|
Etiology |
Non-tuberculous mycobacteria |
Chromoblastomycosis- Dematiaceous fungi Blastomycosis- Blastomyces dermatitidis Cryptococcosis-Cryptococcus neoformans |
Leishmania tropica |
Non-Langerhans cell histiocytosis |
Necrotizing lymphadenitis of unknown cause |
|
Epidemiology |
No age or sex predilection, increased incidence in immunocompromised state |
No age or sex predilection, increased incidence in immunocompromised state |
No age or sex predilection, most prevalent in Middle East and Afghanistan |
Children and young adults, male predilection (Cutaneous form more common in female Asians) |
Young adults, female predilection |
|
Cutaneous features |
Non-specific lesions including cellulitis, abscesses, folliculitis, pustules, ulcers, ecthyma-like lesions, nodules, infiltrated erythematous plaques, scaling plaques, verrucous ulcers, draining sinuses, sporotrichosis-like lesions, panniculitis |
Papules or pustules progressing to warty or vegetating, crusted plaques, ulcers, and hard infiltrated plaques or nodules |
Brown red to brown yellow papules coalescing to form a plaque that appears close to the scar of an old lesion of cutaneous leishmaniasis |
Slow growing, painless, nonpruritic nodules, plaques, or papules with colouration varying from yellow to red to brown |
Facial erythema, eyelid edema, erythematous papules and plaques on face and trunk, leukocytoclastic vasculitis, urticarial or morbilliform rash |
|
Extra-cutaneous features |
Pulmonary disease, lymphadenitis, musculoskeletal infection, disseminated disease |
Pulmonary disease, lymphadenitis, musculoskeletal infection, central nervous system, disseminated disease |
– |
Massive painless cervical lymphadenopathy, fever, night sweats, weight loss |
Tender cervical lymphadenopathy, fever, weight loss, nausea, vomiting, hepatosplenomegaly |
|
Histopathology |
Tuberculoid granulomas with acid fast bacilli |
Mixed granulomatous response with neutrophilic abscesses |
Diffuse dermal granulomatous infiltrate composed of histiocytes, lymphocytes, giant cells and plasma cells Amastigotes few in number or absent |
Dense upper dermal histiocytic infiltration (Positive for S100, CD68, CD163, HAM-56) with scattered multinucleate giant cells and plasma cells. |
Necrotic keratinocytes, karyorrhexis, vacuolar interface change, dermal lymphohistiocytic infiltrate and dermal papillary edema |
|
Treatment |
Combination of antibiotics including fluoroquinolones, macrolides, rifamycins, aminoglycosides depending on sensitivity results |
Azoles, amphotericin B |
Pentavalent antimonial compounds |
Corticosteroids, sirolimus, chemotherapy, |
Corticosteroids |
Treatment for nontuberculous mycobacteria is species-specific. The recommended regimen for the treatment of skin and soft tissue infection caused by Mycobacterium avium complex includes a combination of three drugs: a macrolide (clarithromycin or azithromycin), ethambutol and rifamycin. The optimal duration of treatment is also unknown, but 6–12 months of treatment is usually recommended.3 Since our patient developed ocular toxicity secondary to previous courses of ethambutol, minocycline was employed as an alternative drug based on its effectiveness in pulmonary Mycobacterium avium complex infection.4
Acknowledgement
We are grateful to Dr Tarun Narang; Dr Megha Sharma and Dr Mayur Parkhi for their help in this article.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflict of interest
There are no conflicts of interest.
References
- Treatment of extrapulmonary nontuberculous mycobacterial diseases. Infect Chemother. 2019;51:245-55.
- [CrossRef] [PubMed] [Google Scholar]
- An official ATS/IDSA statement: Diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367-416.
- [CrossRef] [PubMed] [Google Scholar]
- Clarithromycin with minocycline and clofazimine for mycobacterium avium intracellulare complex lung disease in patients without the acquired immune deficiency syndrome GETIM. Groupe d'Etude et de traitement des Infections à mycobactéries. Int J Tuberc Lung Dis. 1998;2:462-70.
- [PubMed] [Google Scholar]





