Translate this page into:
Development of dysplastic nevus during radotinib therapy in patients with chronic myeloid leukemia
2 Department of Hematology, Seoul St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
Correspondence Address:
Hyun Jeong Park
Department of Dermatology, Yeouido St. Mary's Hospital, College of Medicine, The Catholic University of Korea, 62 Yeouido-dong, Youngdeunpo-gu, Seoul, 315-713
Korea
| How to cite this article: Woo YR, Kim JS, Kim DW, Park HJ. Development of dysplastic nevus during radotinib therapy in patients with chronic myeloid leukemia. Indian J Dermatol Venereol Leprol 2017;83:704-707 |
Sir,
Radotinib is a selective BCR-ABL tyrosine kinase inhibitor (TKI) developed for the treatment of chronic myeloid leukemia (CML). Recently, a phase III clinical trial of radotinib has shown good clinical efficacy and safety in the treatment for this condition.[1] We were unable to find any report describing dysplastic nevus associated with radotinib therapy. Herein, we report three cases of eruptive melanocytic nevi (EMN) with dysplastic change in patients with chronic myeloid leukemia, highlighting the possibility of dysplastic nevus after treatment with radotinib.
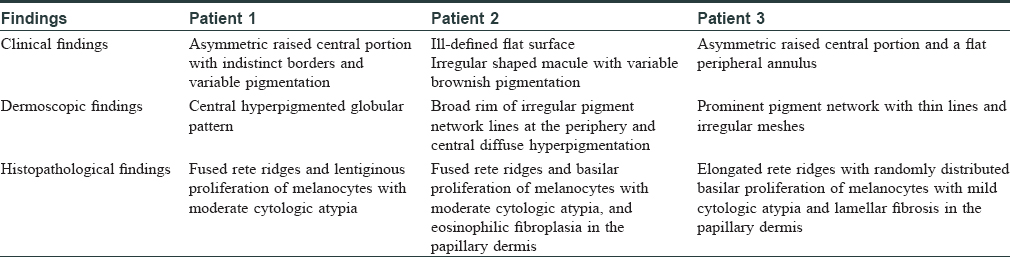
Patient 1 was a 35-year-old male with chronic myeloid leukemia, who began treatment with 800 mg of radotinib in 2013. The patient noticed an increase in the number of pigmented lesions over 1 year. Among them, an atypical pigmented macule on his face exhibited a 5-mm-sized flat surface and asymmetric irregular borders with variable pigmentation [Figure - 1]. Patient 2 was a 59-year-old man diagnosed with chronic myeloid leukemia in 2013 who began treatment with 600 mg radotinib in 2015. One month after treatment with radotinib, he noticed new pigmented macules over his entire body. On physical examination, numerous nevi were observed. Among them, a pigmented macule with asymmetric, irregular, and fuzzy borders was noticed on his face [Figure - 2]. Patient 3 was a 25-year-old man with chronic myeloid leukemia who was prescribed radotinib since 2013. Six months after treatment with radotinib, multiple eruptive nevi with atypical irregular macules were observed on his face and arms [Figure - 3]. All three patients did not receive any immunosuppressive therapies other than radotinib during the therapy. The dermoscopic findings of patient 1, 2, and 3 [Figure - 4] and [Figure - 5] are summarized in [Table - 1]. The biopsies were done for atypical macules for all the three patients. Histopathologic examination of the atypical pigmented lesions of patient 1, 2, and 3 revealed the basilar proliferation of atypical melanocytes and lamellar eosinophilic fibrosis [Figure - 6], [Figure - 7], [Figure - 8]. The nevus cells showed positive immunostaining for HMB-45 and Melan-A [Figure - 9]. In addition, prominent c-kit immunostaining was also observed [Figure - 9]. Based on the clinical, dermoscopic, and histopathological evidence [Table - 1], the three patients were given a final diagnosis of dysplastic nevus with eruptive melanocytic nevi, that developed during radotinib therapy.
 |
| Figure 1: Multiple, pigmented macules on the face in patient 1 |
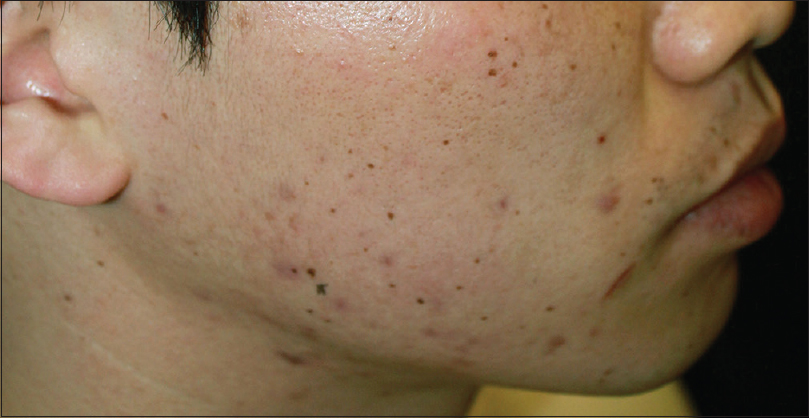 |
| Figure 2: Numerous pigmented macules on the face in patient 2 |
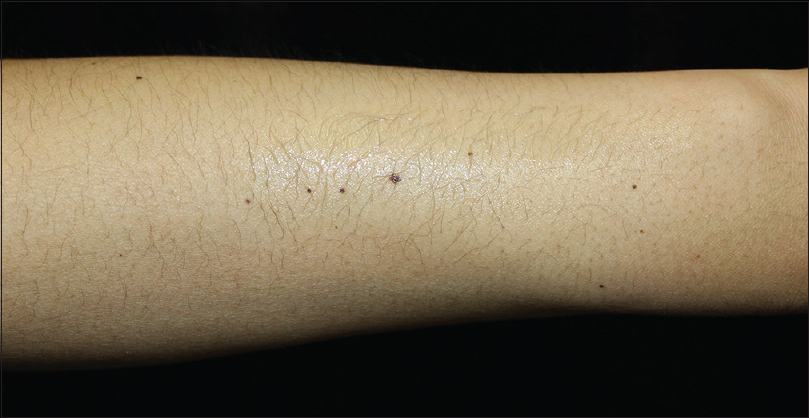 |
| Figure 3: Irregular shaped pigmented macules on the arm in patient 3 |
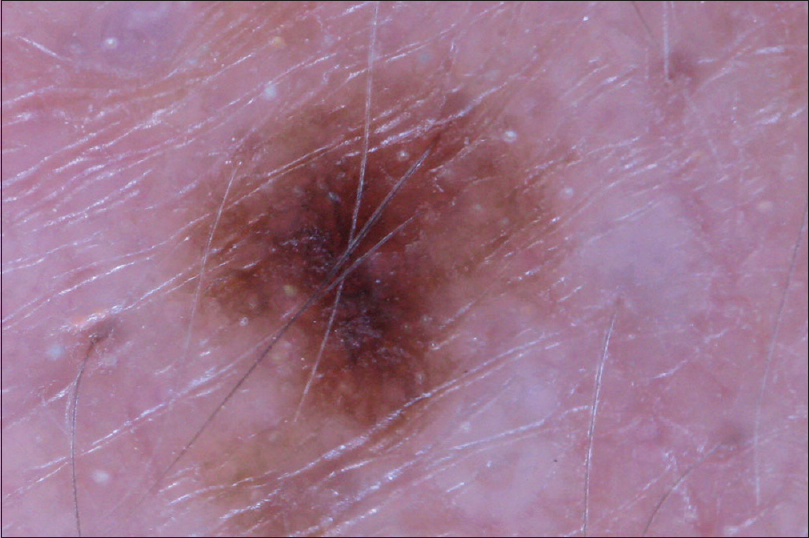 |
| Figure 4: Dermoscopic view of patient 2 shows the dermoscopic view of ill-defined irregular shaped macule with pigment network |
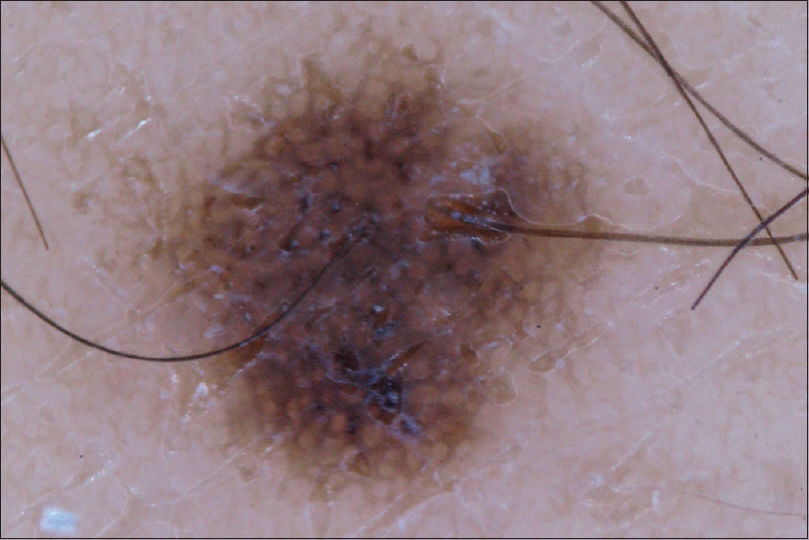 |
| Figure 5: Dermoscopic view of patient 3 shows the atypical macule with prominent pigment network |
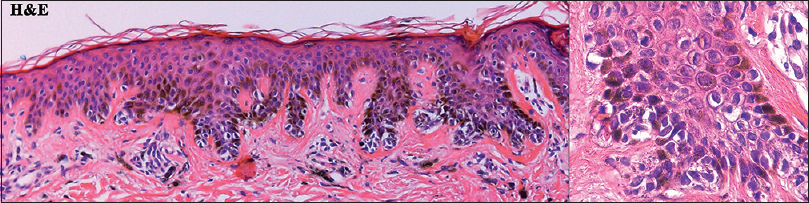 |
| Figure 6: Histopathologic findings of the atypical pigmented macule from patient 1 showed the lentiginous hyperproliferation of melanocytes with moderate cytologic atypia (left panel: H and E, ×100; right panel: H and E, ×400) |
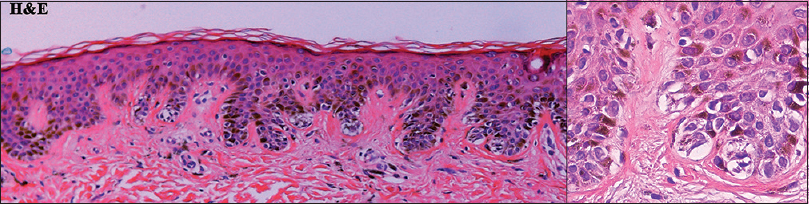 |
| Figure 7: Histopathologic findings from patient 2 showed the basilar proliferation of moderate cytologic atypia of nevus cells (left panel: H and E, ×100; right panel: H and E, ×400) |
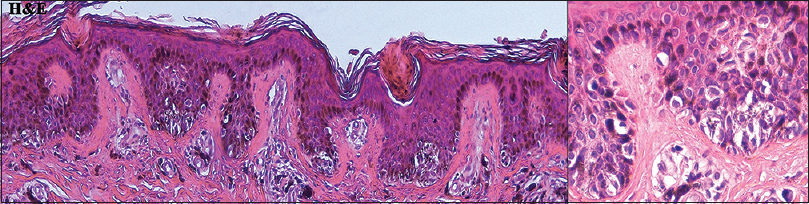 |
| Figure 8: Histopathologic findings from patient 3 showed the basilar proliferation of melanocytes with mild cytologic atypia (left panel: H and E, ×100; right panel: H and E, ×400) |
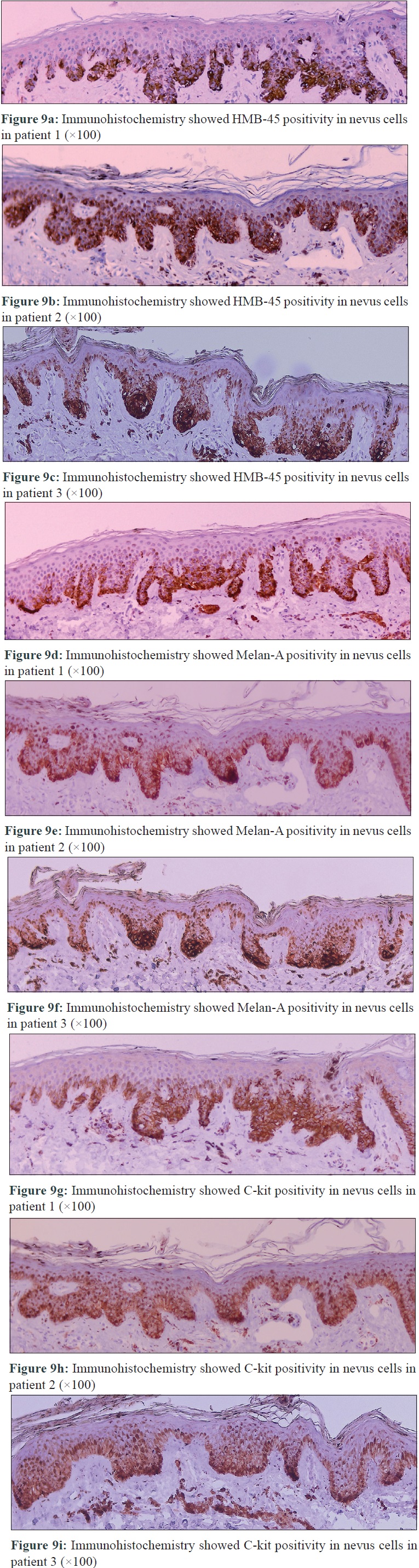 |
| Figure 9 |
Eruptive melanocytic nevi can be associated with various conditions including drug-induced, immunosuppression, and local trauma.[2] The proposed mechanisms for these nevi are not well known; however, some suggest that multiple foci of stimulation or immunosuppression potentially result in disarray in the regulation of melanocyte growth and affect the growth of pigmented lesions. Moreover, the conditions associated with the development of eruptive nevi can also be related to the development of dysplastic nevus.[2]
The tyrosine kinase inhibitors inhibit not only BCR-ABL but also c-kit signalling pathway.[1] Therefore, a well-recognized adverse pigmentary change associated with imatinib is diffuse hypopigmentation. Because the c-kit signalling pathway along with stem cell factors plays an important role in the development of melanocytes, treatment with tyrosine kinase inhibitors results in a significantly decreased number of melanocytes with high tyrosine kinase activity.
However, paradoxical hyperpigmentation has been reported in imatinib and nilotinib-treated patients.[3] In addition, there has been two case reports describing radotinib-induced pigmentary changes, which reported the development of eruptive melanocytic nevi [4] and lentigines.[5] Although the precise mechanism of action for this paradoxical hyperpigmentation has not yet been experimentally demonstrated, we suspect that pigmentary changes after radotinib might be associated with aberrant activation of c-kit in a specific mutant type. Moreover, because chemotherapeutic agents induce cytotoxic effects in epithelial cells, radotinib might exert cytotoxic effects in the epidermal melanocytes and result in dysplastic change of the epidermal melanocytes.[6]
In conclusion, we report the development of dysplastic nevus with eruptive nevi after treatment with radotinib in three patients with chronic myeloid leukemia. After tyrosine kinase inhibitor therapy, the possible occurrence of dysplastic nevus should always be considered. We propose that the cytotoxic effect of radotinib itself and abnormalities in c-kit signalling pathway might result in the development of dysplastic nevi in patients with chronic myeloid leukemia, who had undergone radotinib therapy. Although most dysplastic nevi do not progress into malignant melanoma, there is still a risk of malignant transformation. Therefore, further long-term data are needed to examine the precise risk of development of dysplastic nevus during radotinib therapy and the possible risk of progression to malignant melanoma.
Acknowledgment
This work was supported by the Basic Science Research program and Creative Materials Discovery Program through the National Research Foundation of Korea (NRF), which is funded by the Ministry of Education, Science and Technology and the Ministry of Science, ICT and Future Planning (2015R1C1A2A01055073, 2016M3D1A1021387).
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patients have given their consent for their images and other clinical information to be reported in the journal. The patients understand that name and initials will not be published and due efforts will be made to conceal identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Kwak JY, Kim H, Kim JA, Do YR, Kim HJ, Park JS, et al. Efficacy and safety of radotinib compared with imatinib in newly diagnosed chronic phase chronic myeloid leukemia patients: 12 months result of phase 3 clinical trial. Blood 2015;126:476.
[Google Scholar]
|
| 2. |
Bovenschen HJ, Tjioe M, Vermaat H, de Hoop D, Witteman BM, Janssens RW, et al. Induction of eruptive benign melanocytic naevi by immune suppressive agents, including biologicals. Br J Dermatol 2006;154:880-4.
[Google Scholar]
|
| 3. |
Lee JH, Kang JH, Cho BK, Park HJ. Dysplastic nevus with eruptive melanocytic lesions that developed during nilotinib therapy for chronic myeloid leukemia. Ann Dermatol 2015;27:782-4.
[Google Scholar]
|
| 4. |
Kim M, Yoon YH, Lee JH, Kim DW, Park HJ. Eruptive melanocytic naevi caused by radotinib therapy in patients with chronic myeloid leukaemia: 10 cases and a literature review. Acta Derm Venereol 2017;97:115-6.
[Google Scholar]
|
| 5. |
Won KH, Jo SY, Lee YJ, Chang SE. Radotinib-induced lentiginosis: A report of an adverse cutaneous reaction associated with a tyrosine kinase inhibitor. Clin Exp Dermatol 2016;41:162-5.
[Google Scholar]
|
| 6. |
Susser WS, Whitaker-Worth DL, Grant-Kels JM. Mucocutaneous reactions to chemotherapy. J Am Acad Dermatol 1999;40:367-98.
[Google Scholar]
|
Fulltext Views
2,808
PDF downloads
1,091





