Translate this page into:
Diagnostic utility of plasmacytoid dendritic cells in dermatopathology
-
Received: ,
Accepted: ,
How to cite this article: Bardawil T, Khalil S, Kurban M, Abbas O. Diagnostic utility of plasmacytoid dendritic cells in dermatopathology. Indian J Dermatol Venereol Leprol 2021;87:3-13.
Abstract
Differentiating cutaneous diseases that mimic each other clinically and histopathologically can at times be a challenging task for the dermatopathologist. At the same time, differentiation of entities with overlapping features may be crucial for patient management. Although not seen in normal skin, plasmacytoid dendritic cells usually infiltrate the skin in several infectious, inflammatory/autoimmune and neoplastic entities. Plasmacytoid dendritic cells can be identified in tissue using specific markers such as CD123 and/or blood-derived dendritic cell antigen-2. Plasmacytoid dendritic cells are the most potent producers of type I interferons and their activity may therefore be assessed indirectly in tissue using human myxovirus resistance protein A, a surrogate marker for type I interferon production. In recent years, accumulating evidence has established the utility of evaluating for specific plasmacytoid dendritic cell-related parameters (plasmacytoid dendritic cell content, distribution and clustering and/ or human myxovirus resistance protein A expression) as a diagnostic tool in differentiating cutaneous diseases with overlapping features such as the alopecias, lupus and its mimics, and neoplastic entities. In this review, we provide an update on the current evidence on this topic and on the contexts where this can be a useful adjunct to reach the histopathological diagnosis.
Keywords
Dendritic cell
interferon
lupus erythematosus
plasmacytoid dendritic cell
Introduction
In 1958, Lennert and Remmele coined the term lymphoblasts to refer to a newly recognized cell type with round plasma cell-like/secretory lymphocyte morphology.1 These bone-marrow-derived cells express CD 4, HLA-DR, CD36, CD68, interleukin-3 receptor alpha chain (CD123), blood-derived dendritic cell antigen-2/CD303, and Toll-like receptor 7 and Toll-like receptor 9 within endosomal compartments.2-6 Plasmacytoid dendritic cells, unlike myeloid dendritic cells, do not express myeloid antigens such as CD11b, CD11c, CD13, CD14 and CD33.
Although not seen in normal skin, they have been found to play an important role in the pathogenesis of various cutaneous inflammatory, autoimmune, infectious (especially viral) and neoplastic diseases.5-7 When activated, plasmacytoid dendritic cells are capable of producing large quantities of type I interferons (up to 1,000 times more than other types of cells) which provide antiviral resistance and link innate and adaptive immunity [Figure 1].2-6 In the bone marrow, plasmacytoid dendritic cells arise from common dendritic cell progenitors expressing Flt3 (CD135) receptors. Although the mechanisms are not entirely understood, phosphoinositide 3-kinase-dependent activation of the mechanistic target of rapamycin is a key regulator of this pathway. Transcription factor E2-2 also influences the lineage commitment of a common dendritic cell progenitor en route to its becoming a plasmacytoid dendritic cell.8,9

- Plasmacytoid dendritic cells and their multiple functions: through various mediators, plasmacytoid dendritic cells can promote an antiviral state, enhance natural killer cell activation and effector functions, enhance CD8+ T cell effector functions, CD4+ T cell polarization to T helper 1 (T H 1) cell, cross-prime CD8+ T cells, present antigen to CD4+ T cells, promote T helper 17 (TH17) cell commitment and promote regulatory T cells, influence B cell activation/plasma cell generation, recruit immune cells to inflammatory or infected sites, kill tumor cells and induce viral infected CD4+ T-cell apoptosis
As opposed to other dendritic cells which leave the bone marrow as precursors, plasmacytoid dendritic cells leave the bone marrow as developed cells and transit to the lymphoid organs and peripheral blood. When a plasmacytoid dendritic cell encounters a virus, major histocompatibility complex class I and major histocompatibility complex class II, co-stimulatory molecules CD80, CD86, CD83 and c-c chemokine receptor 7 (CCR7) are upregulated, whereas interferon production gradually decreases, thus initiating the maturation of plasmacytoid dendritic cells. The expression of c-c chemokine receptor 7 stimulates the mature plasmacytoid dendritic cells to migrate to a lymph node and there in turn, interact with and stimulate T cells.10 As demonstrated, at least in psoriasis, recruitment of plasmacytoid dendritic cells to the skin is probably mediated by the chemerin/ChemR23 axis (chemerin being an inflammatory chemotactic protein synthesized mainly by dermal fibroblasts and known to induce in vitro plasmacytoid dendritic cell migration).6
Over the past few decades, plasmacytoid dendritic cells have been gaining attention as a diagnostic tool in differentiating many inflammatory and neoplastic conditions with overlapping features.
Plasmacytoid Dendritic Cell Identification
Among the several markers that have been immunohistochemically utilized for plasmacytoid dendritic cell identification, blood-derived dendritic cell antigen-2/ CD303 and CD123 represent the most specific and sensitive, and as such are the most commonly used.5-7,11-13
Blood-derived dendritic cell antigen-2 is a type II C-type lectin receptor selectively expressed on plasmacytoid dendritic cells.13 It is involved in antigen capture and in regulation of type I interferon production. Accumulating evidence has confirmed that blood-derived dendritic cell antigen-2 is a highly specific marker for normal and neoplastic human plasmacytoid dendritic cells, being selectively and strongly expressed on these cells.13-17 As the α subunit of the heterodimer IL-3 receptor, CD123 plays an important role in regulating cellular proliferation.18 It is highly expressed on the surface of plasmacytoid dendritic cells. However, it should be kept in mind that CD123 can be detected with lower intensity on other cell types such as endothelial cells,12 other subsets of dendritic cells10 and activated macrophages, though plasmacytoid dendritic cells can still be differentiated on hematoxylin and eosin staining from the other CD123+ cell types based on their small- to medium-sized plasma cell-like morphology. On the other hand, blood-derived dendritic cell antigen-2 has not been reported to be expressed by other cell types, but it can be partially lost upon the activation of plasmacytoid dendritic cells in inflammatory processes.11,13
Further, plasmacytoid dendritic cell activity can be indirectly assessed via staining for the human myxovirus resistance protein A, an intracellular protein induced by type I interferons and a key mediator of the interferon-induced antiviral response.10,11,19-21 It is synthesized in a dose-dependent manner, positively regulated by type I interferons and is more stable than interferon-α. Hence its tissue expression is considered to be a surrogate marker of local tissue production of type I interferons, the main source of which are plasmacytoid dendritic cells.6,20,21 Based on these characteristics, cytoplasmic human myxovirus resistance protein A expression has been used in many studies as another parameter in addition to the above more specific plasmacytoid dendritic cell-markers in differentiating some overlapping skin conditions.6,10,15-17,20 Table 1 lists the antibodies/clones used in different studies.10-41
| Marker | Clone/source/approximate price | Reference |
|---|---|---|
| CD123 | 7G3/BD/Biosciences/135$ per 100 tests | 7,8,21-24,30,31,33 |
| 9F5/BD Biosciences/288$ per 50 tests | 27 | |
| Cell Marque, CA, USA/160$ per 100 tests | 19 | |
| BR4MS/Novocastra/450$ for 1 mL vial | 29 | |
| BDCA-2/CD303 | 124B3.13/Dendritics, Lyon/397$ for 100 mil vial | 7,8,12-14,37,38 |
| AC144/Miltenyi Biotech, Germany/96$ per 30 tests | 8 | |
| MxA | M143/Freiburg, Germany/180$ for 50 mil vial | 7,8,12-14,17,33,37,38 |
| CD2AP | Polyclonal/Sigma Aldrich/440$ for 100 mil vial | 29 |
MxA: human myxovirus resistance protein A, BDCA-2: blood-derived dendritic cell antigen-2
Plasmacytoid Dendritic Cell-Related Criteria Used in Diagnostic Evaluation
While different studies had some variation in the systems used to immunohistochemically evaluate the presence of plasmacytoid dendritic cells (mainly by using CD123 or blood-derived dendritic cell antigen-2) and/or their product type I interferon (using myxovirus resistance protein A), they generally had in common the evaluation of plasmacytoid dendritic cell content, clustering and distribution.22-36
For plasmacytoid dendritic cell content, semi-quantitative scoring systems that evaluate plasmacytoid dendritic cells as a percentage of the total mononuclear infiltrate were more commonly used than absolute plasmacytoid dendritic cell counts in most of the studies.11,12,15-17,33-36 Plasmacytoid dendritic cell clusters were defined differently among studies, varying from clusters of at least 5 touching plasmacytoid dendritic cells,23 ≥10 plasmacytoid dendritic cells,15-17,25,26,30 ≥15 touching CD123 + plasmacytoid dendritic cells28 to 20 or more cells.22 Most studies however, defined plasmacytoid dendritic cell clusters as having at least 10 plasmacytoid dendritic cells. Clusters can be easily appreciated at medium and high magnifications (×100 and beyond).
Plasmacytoid dendritic cell distribution patterns (superficial and/or deep, perivascular, perifollicular, perieccrine and/ or at the dermo-epidermal junction) were also an important parameter in the differentiation of some of the entities studied.15-17,33-35
Finally, human myxovirus resistance protein A expression was utilized in a few studies as an additional plasmacytoid dendritic cell-related parameter to aid in the differentiation of some entities.15-17 In these studies, the intensity of human myxovirus resistance protein A staining was mostly evaluated semi-quantitatively as negative, weak/patchy or intense/ diffuse staining within the epidermis and inflammatory cells.
Diagnostic Utility of Plasmacytoid Dendritic Cells
In differentiating the alopecias
Scarring alopecia with predominantly lymphocytic inflammation is characteristic of chronic cutaneous lupus erythematosus, lichen planopilaris, frontal fibrosing alopecia and central centrifugal cicatricial alopecia, all of which at times may be difficult to differentiate.16,22,23
Using blood-derived dendritic cell antigen-2 as specific plasmacytoid dendritic cell marker, a study evaluating 17 lupus erythematosus-associated alopecia, 20 lichen planopilaris and 10 frontal fibrosing alopecia cases (only cases with straightforward clinicopathological features of the entities were included) found that lupus erythematosus-associated alopecia showed increased plasmacytoid dendritic cell content (≥10% plasmacytoid dendritic cells in all cases and ≥50% in 94% of cases), plasmacytoid dendritic cell clusters (100% of cases) and deeper dermal and perieccrine distribution (100% of cases) with the involvement of the dermo-epidermal junction (94% of cases) [Figure 2].16 On the other hand, the majority of lichen planopilaris and frontal fibrosing alopecia cases had <10% plasmacytoid dendritic cell content that was mainly confined to the upper dermis surrounding the hair infundibula, with rare dermo-epidermal junction involvement and rare clustering [Figure 2].16 This study also demonstrated intense and diffuse human myxovirus resistance protein A staining in all cases of lupus erythematosus-associated alopecia, as compared to patchier staining in lichen planopilaris and frontal fibrosing alopecia.
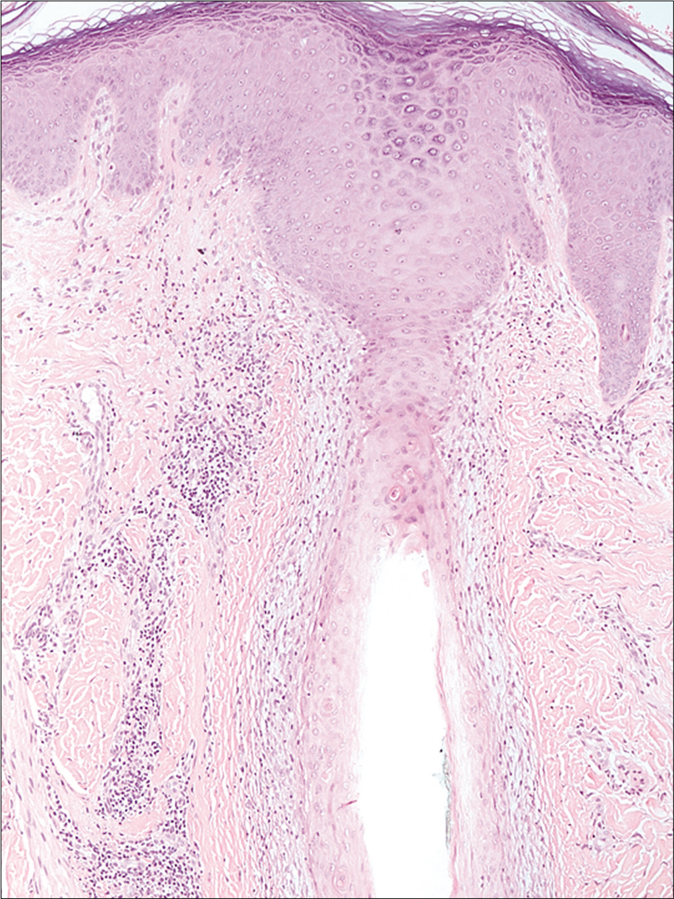
- Lichen planopilaris (H and E,×100)
- CD303/BDCA-2 (clone 124B3.13/Dendritics, Lyon) immunostaining highlights scattered plasmacytoid dendritic cells mainly distributed in peri-infundibular locations in lichen planopilaris (×200)
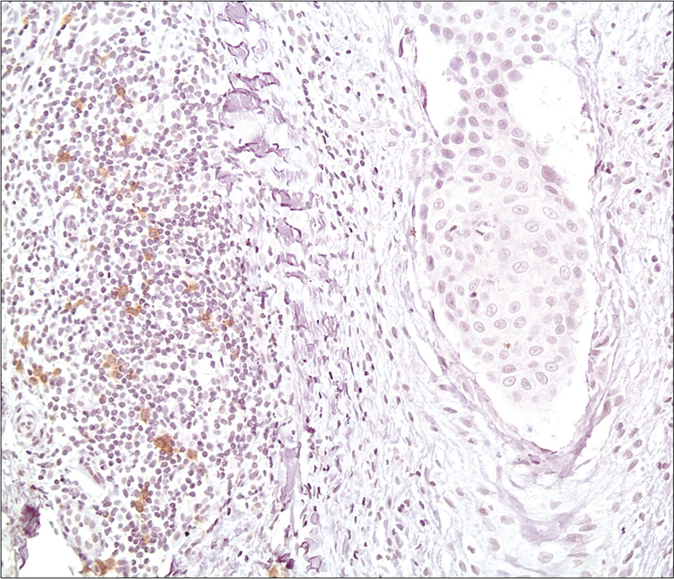
- Lupus-associated alopecia (H and E,×200)
- Numerous plasmacytoid dendritic cells in clusters and as single cells, in superficial and deep locations in cutaneous lupus erythematosus (×100)
Comparable results were demonstrated in two additional studies. In a retrospective study of 35 lichen planopilaris cases and 20 lupus erythematosus cases, clusters of CD123+ plasmacytoid dendritic cells were a reliable histopathological clue highly predictive of lupus erythematosus-associated alopecia rather than lichen planopilaris.23 In another study that included 17 chronic cutaneous lupus erythematosus, 19 lichen planopilaris and 9 central centrifugal cicatricial alopecia cases, a greater percentage of CD123+ plasmacytoid dendritic cells was demonstrated in the infiltrate of chronic cutaneous lupus erythematosus cases (all cases demonstrating at least 10% plasmacytoid dendritic cells and 58.8% of cases showing greater than 20% plasmacytoid dendritic cells) as compared to central centrifugal cicatricial alopecia (33.3% demonstrating at least 10% plasmacytoid dendritic cells and none showing greater than 20% plasmacytoid dendritic cells) and lichen planopilaris (10.5% demonstrating at least 10% plasmacytoid dendritic cells and 5.3% of cases showing greater than 20% plasmacytoid dendritic cells).22 Further, the plasmacytoid dendritic cells were located as clusters of 20 or more cells in chronic cutaneous lupus erythematosus, as compared to their arrangement as single, interstitial cells in lichen planopilaris and central centrifugal cicatricial alopecia.22
As with scarring alopecias, overlapping features between some of the non-scarring alopecias such as alopecia areata, trichotillomania and androgenetic alopecia may make their differentiation difficult.17 One study evaluating 19 alopecia areata, 10 trichotillomania and 7 androgenetic alopecia cases showed that blood-derived dendritic cell antigen-2+ plasmacytoid dendritic cells were more abundant in alopecia areata than in trichotillomania, and that their distribution was different. In alopecia areata, plasmacytoid dendritic cells exclusively occurred in a peribulbar location, while trichotillomania cases showed superficial perivascular and/or peri-follicular plasmacytoid dendritic cells; plasmacytoid dendritic cells were absent in all cases of androgenetic alopecia. Human myxovirus resistance protein A expression too was significantly higher in alopecia areata compared to trichotillomania, and negative in androgenetic alopecia. In addition, intense and diffuse human myxovirus resistance protein A staining of hair bulbs and peribulbar infiltrate was mainly seen in alopecia areata while in trichotillomania, patchy human myxovirus resistance protein A staining mainly occurred in the epidermis, upper follicular epithelium and/or inflammatory infiltrate.23
In differentiating lupus erythematosus from cutaneous lymphoma
Both lupus erythematosus panniculitis and subcutaneous panniculitis-like T-cell lymphoma may present clinically as erythematous nodules or plaques and are histologically characterized by lobular panniculitis and subcutaneous lobular lymphocytic infiltrates, and are sometimes challenging to differentiate.27,28,30
Liau et al. analyzed biopsies from 21 lupus erythematosus panniculitis and 11 subcutaneous panniculitis-like T-cell lymphoma patients using CD123 staining.27 Clusters of plasmacytoid dendritic cells were characteristically seen in the subcutaneous infiltrate of lupus erythematosus panniculitis lesions (17/21, 81%) with moderate to marked staining intensity, especially near lymphoid aggregates. In contrast, such clusters were seen in only 2 of 11 (18.2%) subcutaneous panniculitis-like T-cell lymphoma lesions with the majority (9/11, 82%) containing no or only rare individual plasmacytoid dendritic cells without clustering. The sensitivity and specificity of clusters of plasmacytoid dendritic cells as an adjunctive diagnostic marker for lupus erythematosus panniculitis were 81% and 81.8%, respectively. Moreover, small clusters of plasmacytoid dendritic cells or single plasmacytoid dendritic cells were identified in a peri-adnexal and perivascular distribution in the dermis in 18 lupus erythematosus panniculitis biopsies.
In another study, 22 biopsies from 13 patients with subcutaneous panniculitis-like T-cell lymphoma and 15 biopsies from 7 patients with lupus erythematosus panniculitis were examined.30 CD123 staining revealed plasmacytoid dendritic cell clusters (>10 cells) in 86% of lupus erythematosus panniculitis biopsies evaluated, as opposed to only 13% of subcutaneous panniculitis-like T-cell lymphoma biopsies. Of note, individual cells and smaller aggregates of CD123-positive cells were present in many biopsies from both diseases. The authors of both studies conclude that although clusters may favor lupus erythematosus panniculitis diagnosis, the findings should be interpreted in the context of clinical, histopathologic and molecular findings.
In a more recent study, skin biopsies of cutaneous lupus erythematosus (n = 18), lupus erythematosus panniculitis (n = 17), mycosis fungoides (n = 25) and subcutaneous panniculitis-like T-cell lymphoma (n = 9) were immunostained with CD123.28 Lupus erythematosus panniculitis showed a significantly higher percentage of CD123+ plasmacytoid dendritic cells than subcutaneous panniculitis-like T-cell lymphoma, with plasmacytoid dendritic cell in clusters and comprising ≥20% of the entire infiltrate in the former.28
A study using human myxovirus resistance protein A immunostaining on 5 subcutaneous panniculitis-like T-cell lymphomas, 9 primary cutaneous gamma/delta T-cell lymphoma and 9 lupus erythematosus panniculitis cases showed that minimal human myxovirus resistance protein A staining is a predictor of subcutaneous panniculitis-like T-cell lymphoma or gamma/delta T-cell lymphoma (human myxovirus resistance protein A was primarily seen in macrophages and generally did not exceed 20% of the infiltrate), while extensive human myxovirus resistance protein A staining was a feature of lupus erythematosus panniculitis (36>37 > 50% of the infiltrate staining on average with greater intensity and unique staining of endothelial cells).37
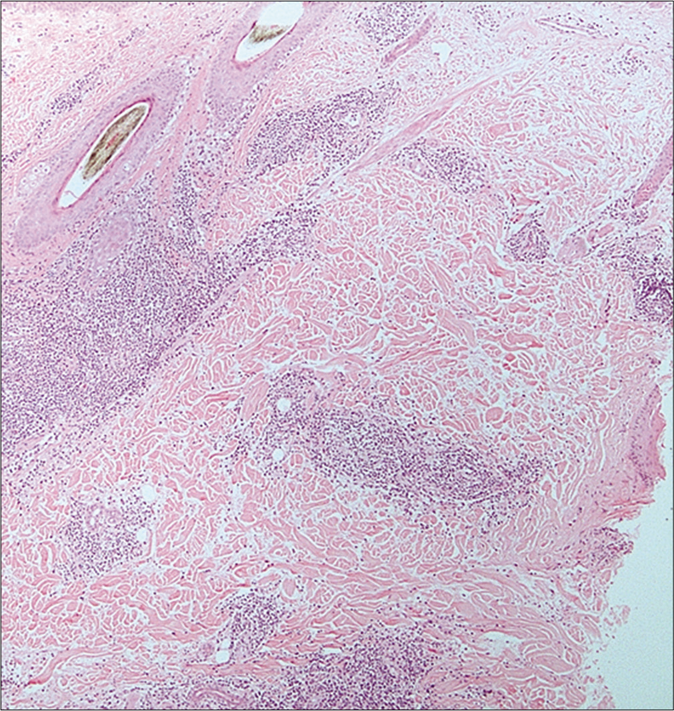
Although mycosis fungoides is readily diagnosed histologically when fully developed, early cases often lack characteristic features such as Pautrier microabscesses. Such cases may mimic various inflammatory dermatoses, notably cutaneous lupus erythematosus.28 In their study, Chen et al. demonstrated a significantly higher percentage of CD123+ plasmacytoid dendritic cells in cutaneous lupus erythematosus than in mycosis fungoides[Figure 3], more frequently comprising ≥20% of the entire infiltrate and forming clusters of ≥15 CD123+ plasmacytoid dendritic cells.28 Recently, Roda et al. described a case of chronic cutaneous lupus erythematosus that showed histopathological features highly suggestive of mycosis fungoides.29 Band-like CD123+ plasmacytoid dendritic cells at the dermo-epidermal junction (>10% of the inflammatory infiltrate) served as a useful clue to the diagnosis of chronic cutaneous lupus erythematosus in addition to the presence of increased dermal mucin deposition and basement membrane zone thickening while lymphoid cytologic atypia and Pautrier microabscesses were absent.
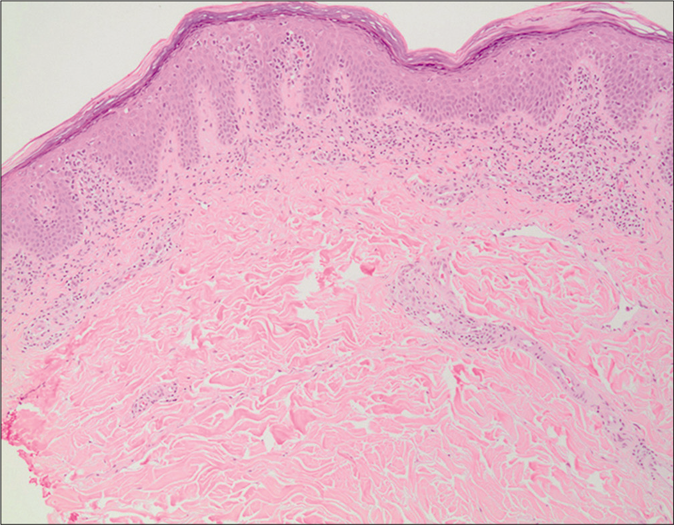
- Mycosis fungoides (H and E,×100)
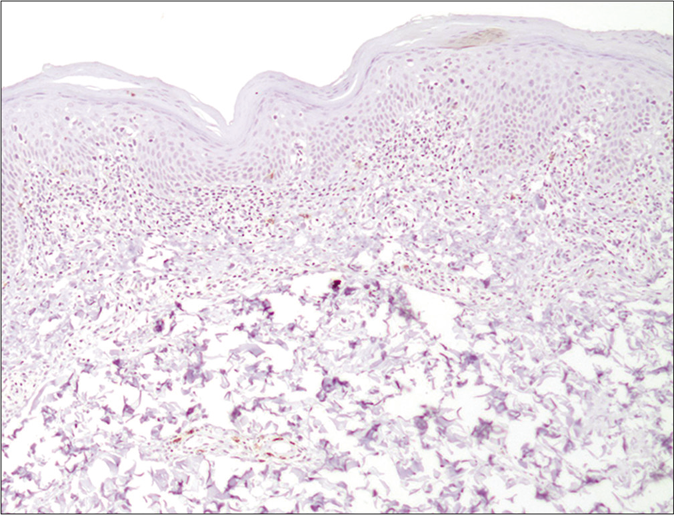
- CD303/BDCA-2 (clone 124B3.13/Dendritics, Lyon) immunostaining highlighting only a few scattered plasmacytoid dendritic cells within the superficial dermal band-like infiltrate in mycosis fungoides (×100)
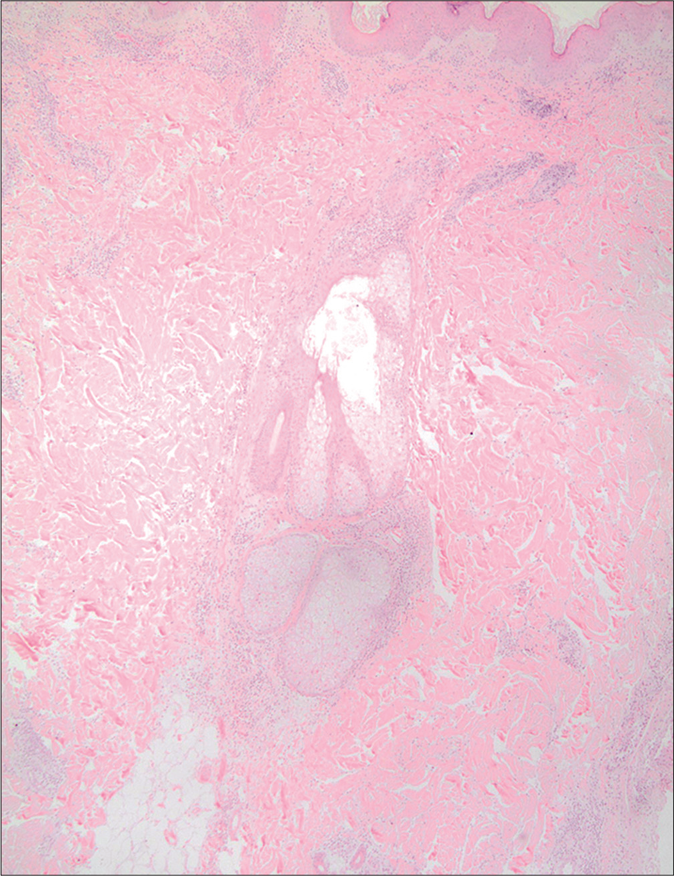
- Cutaneous lupus erythematosus (H and E,×100)

- Numerous plasmacytoid dendritic cells in clusters and as single cells, in superficial and deep locations in cutaneous lupus erythematosus (×100)
In differentiating lupus erythematosus from other mimics
Histopathologically, skin lesions oflupus erythematosus and dermatomyositis are generally indistinguishable.34,35 Examining plasmacytoid dendritic cells in 28 dermatomyositis and 27 lupus erythematosus biopsies, one study demonstrated consistently greater numbersof CD123+ plasmacytoid dendritic cells and larger plasmacytoid dendritic cell percentages in the overall infiltrate in discoid lupus erythematosus (CD123+ cells represented 30% or more of the infiltrate in 12/20 cases) and subacute cutaneous lupus erythematosus lesions (CD123+ cells represented at least 20% of the infiltrate in 5/7 cases) than in dermatomyositis skin lesions (19/28 biopsies showed only rare or up to 5% CD123+ cells in the infiltrate).34 In addition, plasmacytoid dendritic cells in dermatomyositis were preferentially located in the epidermis in contrast to discoid lupus erythematosus and subacute cutaneous lupus erythematosus lesions where plasmacytoid dendritic cells were mainly located in the dermis.34 As for human myxovirus resistance protein A expression, it was generally positive in both lupus erythematosus and dermatomyositis with no significant differences.33,35
Differentiatingutaneous lupus erythematosus from rosacea can sometimes be challenging because of significant overlap clinically (facial erythema or inflammatory papules) and histologically (perifollicular and perivascular lymphocytic infiltrates). In a study that included 30 facial lupus erythematosus and 27 rosacea cases, CD123+ plasmacytoid dendritic cells were more abundant, more likely to form clusters and to comprise at least 20% of the infiltrate in lupus erythematosus than in rosacea.31
Hypertrophic lupus erythematosus may at times be difficult to clinically and histologically distinguish from squamous neoplasms.24,25 In a study by Ko et al., 5 cases of hypertrophic lupus erythematosus and 10 cases of squamous cell carcinoma and hypertrophic actinic keratoses were stained with CD123.24 In all cases of hypertrophic lupus erythematosus, a heavy band of CD123+ cells was found in the upper dermis, clustered at the dermo-epidermal junction. In contrast, only single or rare scattered clusters of CD123+ cells were seen in squamous cell carcinoma and actinic keratoses.
Using CD123 immunostaining, Walsh et al. also showed a significant diagnostic value of the following criteria using plasmacytoid dendritic cells in differentiating hypertrophic lupus erythematosus (27 cases) from its neoplastic (5 cases of actinic keratosis, 5 cases of squamous cell carcinoma in situ, 1 case of keratoacanthoma, and 5 cases of lichenoid keratosis) and inflammatory (5 cases of psoriasis, 5 cases of psoriasiform dermatitis, 9 cases of lichen planus, and 4 cases of granuloma annulare) mimics: representation of ≥10% of inflammatory infiltrate, an arrangement in clusters of ≥10 cells and presence at the dermo-epidermal junction, with accuraciesof 77%, 74% and 71%, respectively.25
Tomasini et al. also reported that all their 54 cases of discoid lupus erythematosus showed a similar band of CD123+ cells at the dermo-epidermal junction, and prominent clusters of CD123+ cells were also found around vessels and adnexal structures in them.26 In contrast, all 4 cases of squamous cell carcinoma and all 2 cases of actinic keratoses showed only scattered CD123+ cells in the infiltrate.
In differentiating pustular psoriasis from acute generalized exanthematous pustulosis
Acute generalized exanthematous pustulosis is a reaction pattern that most commonly occurs secondary to medications.36 It closely resembles pustular psoriasis clinically and histopathologically. It has also been suggested that acute generalized exanthematous pustulosis may represent a variant or acute exacerbation of pustular psoriasis. Vyas et al. stained 43 skin biopsies from cases of acute generalized exanthematous pustulosis (18 cases) and pustularpsoriasis (25 cases) with CD123, 26 of which were also stained for human myxovirus resistance protein A. A significant number of pustular psoriasis cases showed higher numbers of dermal (24/25) and epidermal (11/25) plasmacytoid dendritic cells compared to acute generalized exanthematous pustulosis (only 5/18 cases showing dermal cells and 2/18 cases showing epidermal cells) cases. Perivascular and intraepidermal plasmacytoid dendritic cells, dilated tortuous vessels, and human myxovirus resistance protein A expression in the dermal inflammatory infiltrate significantly favored a diagnosis of pustular psoriasis, whereas the absence of plasmacytoid dendritic cells and presence of eosinophils significantly favored a diagnosis of acute generalized exanthematous pustulosis.36
In differentiating cutaneous lymphoproliferative disorders
CD30-positive lymphoproliferative disorders
Primary cutaneous CD30-positive lymphoproliferative disorders include lymphomatoid papulosis and primary cutaneous anaplastic large T-cell lymphoma. Clinical and histopathological simulants include reactive benign CD30-positive conditions such as arthropod bite reactions and viral infections such as molluscum contagiosum. De Souza et al. assessed the composition of the tumor microenvironment in skin biopsies from 18 cases of lymphomatoid papulosis, 8 of cutaneous anaplastic large T-cell lymphoma and 18 of reactive diseases harboring CD30+ cells. Their findings revealed that CD30-positive reactive inflammatory and infectious disorders were characterized by significantly higher numbers of CD123+ CD2AP+ (CD 2 adaptor protein) plasmacytoid dendritic cells (6.3%) when compared to lymphomatoid papulosis (1%) and primary cutaneous anaplastic large T-cell lymphoma (1.1%).32
Cutaneous B-cell lymphomas
Assessment for plasmacytoid dendritic cells in cutaneous marginal zone B-cell lymphoma and other primary cutaneous B-cell lymphoproliferative disorders has also been investigated.38,39 Clusters containing more than 10 CD123+ plasmacytoid dendritic cells were demonstrated in all 23 cases of cutaneous marginal zone B-cell lymphoma cases compared to only a minority (4 of 31; 13%) of primary cutaneous follicle center lymphoma and none of 12 cases of primary cutaneous diffuse large B-cell lymphoma, leg type.38 Plasmacytoid dendritic cell clusters in cutaneous marginal zone B-cell lymphoma were large and occurred close to monoclonal plasma cells and T-cells. In addition to implying a pathogenetically relevant role of the plasmacytoid dendritic cells in cutaneous marginal zone B-cell lymphoma, the authors concluded that plasmacytoid dendritic cells may be used as an additional diagnostic marker for cutaneous marginal zone B-cell lymphoma.
In differentiating keratoacanthoma from squamous cell carcinoma
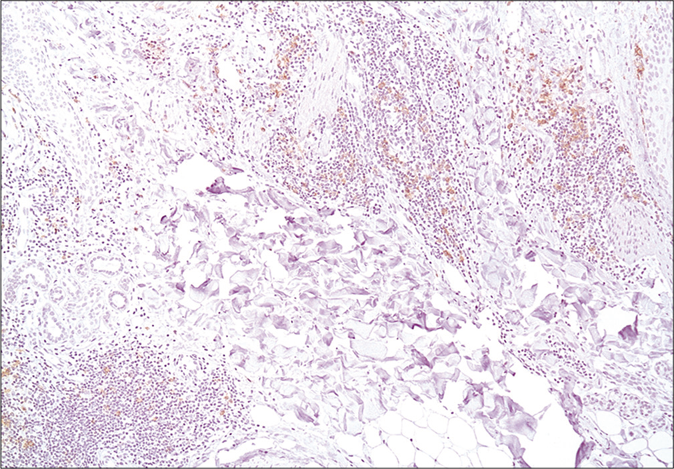
Controversy still exists whether keratoacanthoma is a benign crateriform epithelial neoplasm prone to spontaneous regression, or a variant of well-differentiated squamous cell carcinoma. In assessing for plasmacytoid dendritic cells and human myxovirus resistance protein A expression in 20 keratoacanthomas versus 20 squamous cell carcinoma cases, Abbas et al. found that blood-derived dendritic cell antigen-2+ plasmacytoid dendritic cells were present in 100% of keratoacanthomas and 90% of squamous cell carcinoma cases.15 Plasmacytoid dendritic cells were also more abundant (comprising more than 10% of the infiltrate in 19/20 cases of keratoacanthoma versus 11/20 cases of squamous cell carcinoma), formed clusters (12/20 cases of keratoacanthoma versus 2/20 cases of squamous cell carcinoma) and infiltrated neoplastic epithelium (10/20 cases of keratoacanthoma versus 1/20 caess of squamous cell carcinoma) significantly more in the keratoacanthomas than in the squamous cell carcinomas [Figure 4]. Further, human myxovirus resistance protein A expression was also significantly greater in keratoacanthomas than in squamous cell carcinomas (intense diffuse expression in 19/20 cases of keratoacanthoma versus 4/20 cases of squamous cell carcinoma) [Figure 4]. According to the authors, these findings support a possible role for plasmacytoid dendritic cells in keratoacanthoma pathogenesis and regression.
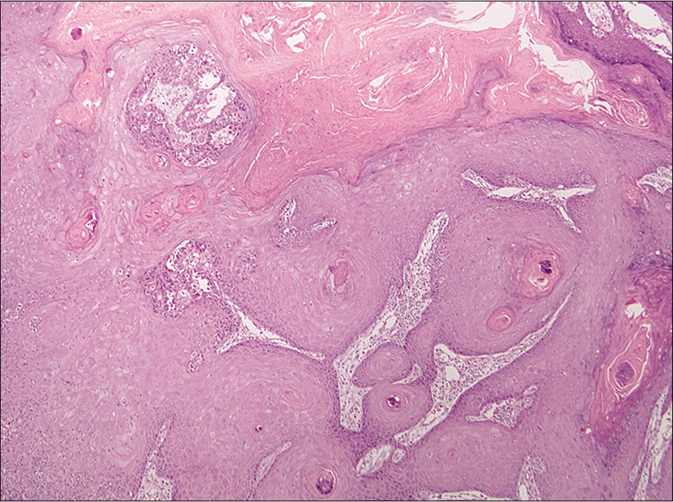
- Keratoacanthoma (H and E, ×100)
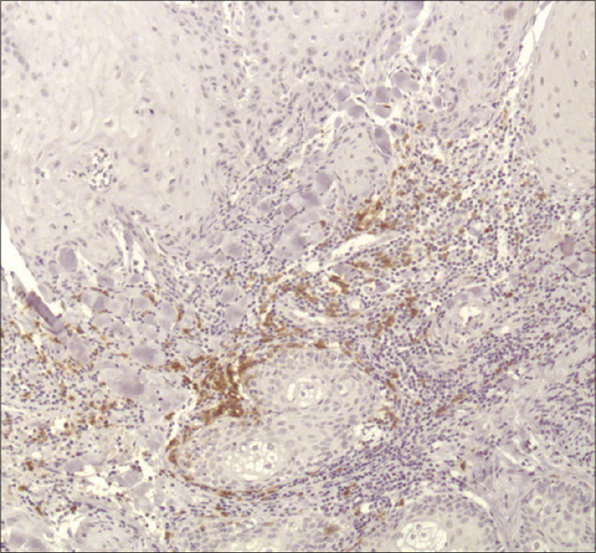
- CD303/BDCA-2 (clone 124B3.13/Dendritics, Lyon) immunostaining highlights the presence of numerous plasmacytoid dendritic cells as single cells and clusters in keratoacanthoma (×100)
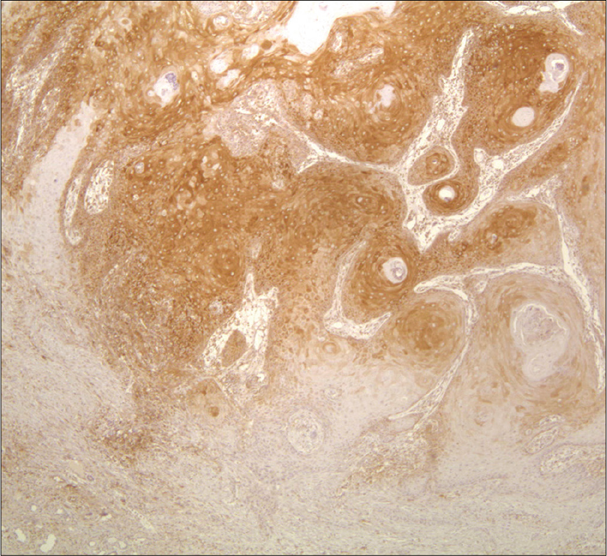
- Human myxovirus resistance protein A (clone M143/Freiburg, Germany) immunostaining is diffuse and intense in keratoacanthoma (×100)
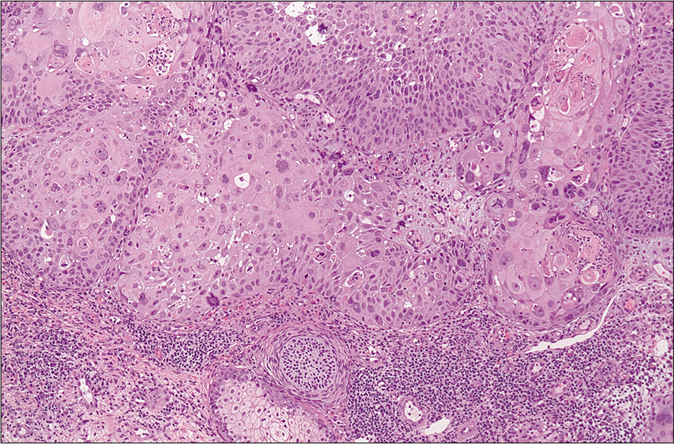
- Squamous cell carcinoma (H and E, × 200)

- Only a few plasmacytoid dendritic cells present singly cells in squamous cell carcinoma (×100)
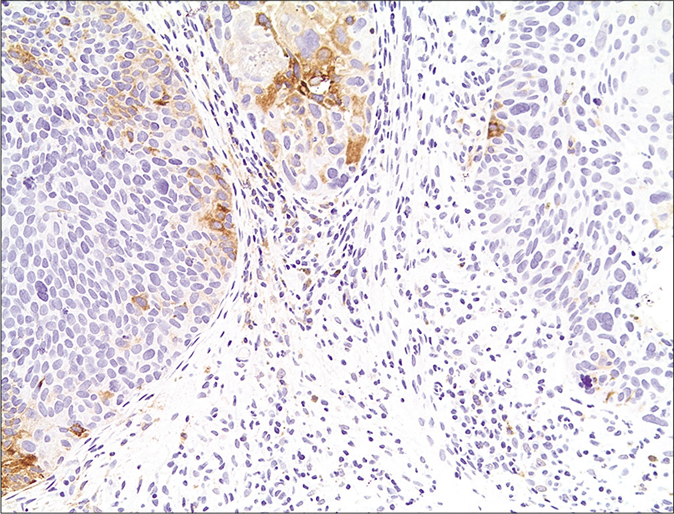
- Weak and patchy myxovirus resistance protein A immunostaining in squamous cell carcinoma (×100)
Other studies
Several attempts using plasmacytoid dendritic cells to differentiate other entities have been reported but these showed either negative findings or controversial results. No significant differences were found when comparing plasmacytoid dendritic cells in lichen planus versus lichen striatus,40 lichen planus versus pityriasis lichenoides,41 graft-versus-host disease versus drug eruptions,42 lupus erythematosus versus polymorphous light eruption,43,44 and lupus erythematosus versus perniosis.45
Limitations of Plasmacytoid Dendritic Cell Use
The main limitations to the use of plasmacytoid dendritic cell as a diagnostic tool would be the additional immunostaining cost, the availability of relevant antibodies locally, and the expertise needed to use the stains appropriately and then to interpret the plasmacytoid dendritic cell-related parameters in various case scenarios.
Main limitations to the review is that many of the studies done included small number of cases and many also included only straightforward cases of the different compared entities, while excluding the more cofusing cases with overlapping features.
Conclusion
The available literature establishes the role of plasmacytoid dendritic cells as a useful diagnostic tool in differentiating challenging cutaneous diseases that may otherwise mimic each other clinically and histopathologically [Table 2]. This is especially true when differentiating the skin lesions in the different presentations of lupus erythematosus from their mimics. Though the distinction remains challenging and careful clinicopathologic correlation is always necessary, CD123/ blood-derived dendritic cell antigen-2 and human myxovirus resistance protein A immunostaining for the presence or absence of plasmacytoid dendritic cells may help differentiate certain cutaneous conditions, and may be used with ancillary studies such as regular stains (e.g., mucin stains or PAS for checking on basement membrane thicking in LE) or serological or molecular testing to reach the diagnosis. Further studies are needed to confirm the diagnostic value of the above observations and to strengthen the argument for the assessment of plasmacytoid dendritic cells in regular practice.
| Clinical entities | Plasmacytoid dendritic cell parameters |
|---|---|
| Scarring alopecias | lupus erythematosus_associated alopecia: all ≥10% plasmacytoid dendritic cell content, plasmacytoid dendritic cell clusters in all cases and superficial and deeper dermal and perieccrine distribution(100% of cases) with involvement of the dermo-epidermal junction, intense and diffuse myxovirus resistance protein A staining lichen planopilaris and frontal fibrosing alopecia: majority <10% plasmacytoid dendritic cell content, rare plasmacytoid dendritic cell clusters, upper dermal distribution surrounding hair infundibula with rare dermo-epidermal junction involvement, mostly patchy myxovirus resistance protein A staining central centrifugal cicatricial alopecia: majority <10% plasmacytoid dendritic cell content, rare plasmacytoid dendritic cell clusters |
| alopecia areata versus trichotillomania | plasmacytoid dendritic cells more abundant and myxovirus resistance protein A expression significantly higher in alopecia areatathan in trichotillomania alopecia areata: plasmacytoid dendritic cells in peribulbar location, intense and diffuse myxovirus resistance protein A staining of hair bulbs and peribulbar infiltrate Trichotillomania: superficial perivascular and/or peri-follicular plasmacytoid dendritic cells , patchy myxovirus resistance protein A staining mainly in the epidermis, upper follicular epithelium and/or inflammatory infiltrate |
| lupus erythematosus panniculitis versus subcutaneous panniculitis-like T-cell lymphoma | lupus erythematosus panniculitis: most show subcutaneous plasmacytoid dendritic cell clusters, peri-adnexal/perivascular dermal single plasmacytoid dendritic cells or in small clusters, extensive myxovirus resistance protein A staining subcutaneous panniculitis-like T-cell lymphoma: the majority show no or only rare individual plasmacytoid dendritic cells without clustering. Also, minimal myxovirus resistance protein A staining |
| cutaneous lupus erythematosus versus mycosis fungoides | Significantly higher plasmacytoid dendritic cell percentage and clustering in cutaneous lupus erythematosus than mycosis fungoides |
| lupus erythematosus and dermatomyositis | Consistently greater plasmacytoid dendritic cell content in lupus erythematosus and subacute cutaneous lupus erythematosus lesions than in dermatomyositis plasmacytoid dendritic cells in dermatomyositis preferentially located in the epidermis in contrast to lupus erythematosus and subacute cutaneous lupus erythematosus lesions where plasmacytoid dendritic cells mainly located in the dermis myxovirus resistance protein A expression positive in both lupus erythematosus and dermatomyositis |
| cutaneous lupus erythematosus versus rosacea | plasmacytoid dendritic cells more abundant and more likely to form clusters and to comprise at least 20% of the infiltrate in lupus erythematosus than in rosacea |
| Hypertrophic lupus erythematosus versus squamous neoplasms | Hypertrophic lupus erythematosus : heavy plasmacytoid dendritic cell band in the upper dermis, clustered at dermo-epidermal junction squamous cell carcinomaand actinic keratosis: only single or rare scattered clusters of plasmacytoid dendritic cells |
| acute generalized exanthematous pustulosis versus pustular psoriasis | Perivascular and intraepidermal plasmacytoid dendritic cells and myxovirus resistance protein A expression in the dermal inflammatory infiltrate significantly favored pustular psoriasis plasmacytoid dendritic cell absence and presence of eosinophils significantly favored acute generalized exanthematous pustulosis |
| lymphomatoid papulosis/primary cutaneous anaplastic large T-cell lymphoma versus reactive CD30-positive disorders | CD30-positive reactive disorders had significantly higher plasmacytoid dendritic cell numbers compared to lymphomatoid papulosis and primary cutaneous anaplastic large T-cell lymphoma |
| cutaneous marginal zone B-cell lymphoma versus primary cutaneous B-cell lymphoproliferative disorders | plasmacytoid dendritic cell clusters in all cutaneous marginal zone B-cell lymphoma cases compared to only a minority of primary cutaneous follicle center lymphoma and none of primary cutaneous diffuse large B-celllymphoma, leg type |
| keratoacanthoma versus squamous cell carcinoma | plasmacytoid dendritic cells more abundant, formed clusters and infiltrated neoplastic epithelium significantly more in keratoacanthoma than in squamous cell carcinoma myxovirus resistance protein A expression significantly greater in keratoacanthoma than in squamous cell carcinoma |
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patients have given their consent for their images and other clinical information to be reported in the journal. The patient understands that name and initials will not be published and due efforts will be made to conceal identity but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Karyometric research on lymph node cells in man. I. Germinoblasts, lymphoblasts& lymphocytes. Acta Haemato. 1958;19:99-113.
- [CrossRef] [PubMed] [Google Scholar]
- Plasmacytoid dendritic cells in immunity. Nat Immunol. 2004;5:1219-26.
- [CrossRef] [PubMed] [Google Scholar]
- Plasmacytoid dendritic cells: Sensing nucleic acids in viral infection and autoimmune diseases. Nat Rev Immunol. 2008;8:594-606.
- [CrossRef] [PubMed] [Google Scholar]
- The multifaceted biology of plasmacytoid dendritic cells. Nat Rev Immunol. 2015;15:471-85.
- [CrossRef] [PubMed] [Google Scholar]
- Plasmacytoid dendritic cell role in cutaneous malignancies. J Dermatol Sci. 2016;83:3-9.
- [CrossRef] [PubMed] [Google Scholar]
- Update on the role of plasmacytoid dendritic cells in inflammatory/autoimmune skin diseases. Exp Dermatol. 2016;25:415-21.
- [CrossRef] [PubMed] [Google Scholar]
- Spontaneous regression of highly immunogenic Molluscumcontagiosum virus (MCV)-induced skin lesions is associated with plasmacytoid dendritic cells and IFN-DC infiltration. J Invest Dermatol. 2011;131:426-34.
- [CrossRef] [PubMed] [Google Scholar]
- Plasmacytoid dendritic cells: Recent progress and open questions. Annu Rev Immunol. 2011;29:163-83.
- [CrossRef] [PubMed] [Google Scholar]
- Antigen-presentation properties of plasmacytoid dendritic cells. Immunity. 2008;29:352-61.
- [CrossRef] [PubMed] [Google Scholar]
- Plasmacytoid dendritic cells: linking innate and adaptive immunity. J Virol. 2004;791:17-27.
- [CrossRef] [PubMed] [Google Scholar]
- Cutaneous distribution of plasmacytoid dendritic cells in lupus erythematosus, Selective tropism at the site of epithelial apoptotic damage. Immunobiology. 2009;214:877-86.
- [CrossRef] [PubMed] [Google Scholar]
- Dendritic cell ontogeny: A human dendritic cell lineage of myeloid origin. Proc Natl AcadSci U S A. 1997;94:12551-6.
- [CrossRef] [PubMed] [Google Scholar]
- BDCA-2, a novel plasmacytoid dendritic cell-specific type II C-type lectin, mediates antigen capture and is a potent inhibitor of interferon alpha/beta induction. J Exp Med. 2001;194:1823-34.
- [CrossRef] [PubMed] [Google Scholar]
- BDCA-2 (CD303): A highly specific marker for normal and neoplastic plasmacytoid dendritic cells. Blood2013;. ;122:296-7.
- [CrossRef] [PubMed] [Google Scholar]
- Plasmacytoid dendritic cell involvement in the host response against keratoacanthoma. J Am Acad Dermatol. 2014;70:1142-5.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of the diagnostic value of plasmacytoid dendritic cells in differentiating the lymphocytic cicatricialalopecias. Dermatology. 2015;231:158-63.
- [CrossRef] [PubMed] [Google Scholar]
- Plasmacytoid dendritic cells in alopecia areata: Missing link? J Eur Acad Dermatol Venereol. 2016;30:119-23.
- [CrossRef] [PubMed] [Google Scholar]
- CD123 (interleukin 3 receptor alpha chain) J Biol Regul Homeost Agents. 2001;15:98-100.
- [Google Scholar]
- Expression of MxA protein in inflammatory dermatoses. J Histochem Cytochem. 1995;43:47-52.
- [CrossRef] [PubMed] [Google Scholar]
- Plasmacytoid dendritic cells (natural interferon-alpha/beta-producing cells) accumulate in cutaneous lupus erythematosus lesions. Am J Pathol. 2001;159:237-43.
- [CrossRef] [Google Scholar]
- Human MxA protein: An interferon-induced dynamin-like GTPase with broad antiviral activity. J Interferon Cytokine Res. 2011;31:79-87.
- [CrossRef] [PubMed] [Google Scholar]
- CD123 immunohistochemistry for plasmacytoid dendritic cells is useful in the diagnosis of scarring alopecia. J Cutan Pathol. 2016;43:643-8.
- [CrossRef] [PubMed] [Google Scholar]
- Clusters of CD123+plasmacytoid dendritic cells help distinguish lupus alopecia from lichen planopilaris. J Am Acad Dermatol. 2016;74:1267-9.
- [CrossRef] [PubMed] [Google Scholar]
- Hypertrophic lupus erythematosus: The diagnostic utility of CD123 staining. J Cutan Pathol. 2011;38:889-92.
- [CrossRef] [PubMed] [Google Scholar]
- Plasmacytoid dendritic cells in hypertrophic discoid lupus erythematosus: An objective evaluation of their diagnostic value. J CutanPathol. 2015;42:32-8.
- [CrossRef] [PubMed] [Google Scholar]
- Plasmacytoid dendritic cells: An overview of their presence and distribution in different inflammatory skin diseases, with special emphasis on Jessner's lymphocytic infiltrate of the skin and cutaneous lupus erythematosus. J Cutan Pathol. 2010;37:1132-9.
- [CrossRef] [PubMed] [Google Scholar]
- The presence of clusters of plasmacytoid dendritic cells is a helpful feature for differentiating lupus panniculitis from subcutaneous panniculitis-like T-cell lymphoma. Histopathology. 2013;62:1057-66.
- [CrossRef] [PubMed] [Google Scholar]
- Utility of CD123 immunohistochemistry in differentiating lupus erythematosus from cutaneous T cell lymphoma. Histopathology. 2019;74:908-16.
- [CrossRef] [PubMed] [Google Scholar]
- Lupus erythematosus mimicking mycosis fungoides: CD123(+) plasmacytoid dendritic cells as a useful diagnostic clue. J CutanPathol. 2019;46:167-70.
- [CrossRef] [PubMed] [Google Scholar]
- Useful Parameters for Distinguishing Subcutaneous Panniculitis-like T-Cell Lymphoma From Lupus Erythematosus Panniculitis. Am J SurgPathol. 2016;40:745-54.
- [CrossRef] [PubMed] [Google Scholar]
- Comparative analysis of rosacea and cutaneous lupus erythematosus: Histopathologic features, T-cell subsets, and plasmacytoid dendritic cells. J Am AcadDermatol2014;. ;71:100-7.
- [CrossRef] [PubMed] [Google Scholar]
- Characterization of the tumor microenvironment in primary cutaneous CD30-positive lymphoproliferative disorders: A predominance of CD163-positive M2 macrophages. J Cutan Pathol. 2016;43:579-88.
- [CrossRef] [PubMed] [Google Scholar]
- Type I interferon-associated skin recruitment of CXCR3+lymphocytes in dermatomyositis. Clin Exp Dermatol. 2006;31:576-82.
- [CrossRef] [PubMed] [Google Scholar]
- Plasmacytoid dendritic cells are present in cutaneous dermatomyositis lesions in a pattern distinct from lupus erythematosus. J CutanPathol. 2008;35:452-6.
- [CrossRef] [PubMed] [Google Scholar]
- The phenotypic profile of dermatomyositis and lupus erythematosus: A comparative analysis. J CutanPathol2010;. ;37:659-71.
- [CrossRef] [PubMed] [Google Scholar]
- Distinguishing pustular psoriasis and acute generalized exanthematouspustulosison the basis ofplasmacytoid dendritic cells and MxA protein. J Cutan Pathol. 2019;46:317-26.
- [CrossRef] [PubMed] [Google Scholar]
- Human myxovirus resistance protein 1 (MxA) as a useful marker in the differential diagnosis of subcutaneous lymphoma vs. lupus erythematosus profundus. Eur J Dermatol. 2012;22:629-33.
- [CrossRef] [PubMed] [Google Scholar]
- CD123-positive plasmacytoid dendritic cells in primary cutaneous marginal zone B-cell lymphoma: Diagnostic and pathogenetic implications. Am J Surg Pathol. 2009;33:1307-13.
- [CrossRef] [PubMed] [Google Scholar]
- CD123-positive plasmacytoid dendritic cells in primary cutaneous marginal zone B-cell lymphoma: A crucial role and a new lymphoma paradigm. Am J Dermatopathol. 2010;32:194-6.
- [CrossRef] [PubMed] [Google Scholar]
- Plasmacytoid Dendritic Cells and Type I Interferon Signature in Lichen Striatus. Pediatr Dermatol. 2016;33:301-6.
- [CrossRef] [PubMed] [Google Scholar]
- Possible role of plasmacytoid dendritic cells in pityriasislichenoides. Clin Exp Dermatol. 2018;43:404-9.
- [CrossRef] [PubMed] [Google Scholar]
- Acute cutaneous graft-vs.-host disease compared to drug hypersensitivity reaction with vacuolar interface changes: A blinded study of microscopic and immunohistochemical features. J Cutan Pathol. 2015;42:39-45.
- [CrossRef] [PubMed] [Google Scholar]
- Plasmacytoid dendritic cells are absent in skin lesions of polymorphic light eruption. Photodermatol Photoimmunol Photomed. 2007;23:24-8.
- [CrossRef] [PubMed] [Google Scholar]
- Cutaneous infiltration of plasmacytoid dendritic cells and T regulatory cells in skin lesions of polymorphic light eruption. J Eur Acad Dermatol Venereol. 2018;32:985-91.
- [CrossRef] [PubMed] [Google Scholar]
- Comparative analysis of chilblain lupus erythematosus and idiopathic perniosis: Histopathologic features and immunohistochemistry for CD123 and CD30. Am J Dermatopathol. 2018;40:265-71.
- [CrossRef] [PubMed] [Google Scholar]






