Translate this page into:
Diffuse alopecia areata is associated with intense inflammatory infiltration and CD8+ T cells in hair loss regions and an increase in serum IgE level
2 Department of Pathology, First Affiliated Hospital of Sun Yat-sen University, China
Correspondence Address:
Xingqi Zhang
Department of Dermatology, First Affiliated Hospital of Sun Yat-sen University, Zhongshan 2nd Road, Guangzhou 510080
China
| How to cite this article: Zhao Y, Zhang B, Caulloo S, Chen X, Li Y, Zhang X. Diffuse alopecia areata is associated with intense inflammatory infiltration and CD8+ T cells in hair loss regions and an increase in serum IgE level. Indian J Dermatol Venereol Leprol 2012;78:709-714 |
Abstract
Background: Mechanism leading to an abrupt hair loss in diffuse alopecia areata (AA) remains unclear. Aims: To explore the characteristics of diffuse AA and possible factors involved in its pathogenesis. Methods: Clinical and laboratory data of 17 diffuse AA patients and 37 patchy AA patients were analyzed retrospectively. Serum IgE level was evaluated in all diffuse and patchy AA patients, as well as 27 healthy subjects without hair loss to serve as normal control. Univariate analysis was performed using Fisher's exact test and Wilcoxon rank-sum test. Associations between inflammatory cell infiltration and laboratory values were analyzed using Spearman rank correlation test. Results: The mean age of patients with diffuse AA was 27 years with a mean disease duration of 1.77 months. All of them presented in spring or summer with an acute onset of diffuse hair loss preceded by higher incidence of scalp pruritus. Although no statistically significant difference on the incidence of atopic disease among three groups has been found, serum IgE level in diffuse AA was higher than that in healthy controls, but was comparable to that in patchy AA group. Histopathology of lesional scalp biopsies showed more intense infiltration comprising of mononuclear cells, eosinophils, CD3 + , and CD8 + T cells around hair bulbs in diffuse AA group than in patchy AA group. Moreover, IgE level in diffuse AA patients positively correlated with intensity of infiltration by mononuclear cells, eosinophils, and CD8 + T cells. Conclusions: Hypersensitivity may be involved in pathogenesis of diffuse AA. The acute onset of diffuse AA may be related to intense local inflammatory infiltration of hair loss region and an increase in serum IgE level.Introduction
Diffuse alopecia areata (AA) is described as a unique AA that lacks the characteristic patches of AA and instead, demonstrates widespread scalp hair thinning. [1] This type of AA can at times be difficult to diagnose as it can easily be confused with other forms of non-scarring alopecia.
However, mechanism leading to this abrupt and diffuse type of hair loss remains unclear. Our objective was to explore the characteristics of diffuse AA and possible factors involved in its pathogenesis by a retrospective comparative analysis of clinical, dermoscopic, histopathological, and serological data of diffuse AA patients with those of patchy AA patients.
Methods
Patients
Between July 2009 and March 2011, seventeen cases of diffuse AA were identified among 524 patients with various types of AA who presented to our hair clinic. Inclusion criteria were patients with diffuse and severe hair thinning which occurred within a few months and lack the characteristic patches of alopecia; histopathology of scalp biopsies showed typical features of AA. Exclusion criteria were patients with history of psychological disease, systemic diseases, nutritional deficiency, or history of drug intake. Thirty-seven age-matched cases of patchy AA patients (comprising of single- and multiple-patch AA) with similar gender ratio and comparable disease duration were enrolled as control group.
Methods
Data collected included age, gender, clinical presentation, duration of disease, family history of AA, concomitant autoimmune diseases or atopy, hair-pull test findings, and response to treatment. Scalp dermoscopic examination of hair-loss regions of 15 patients from the diffuse AA group and all patients from patchy AA group were performed using a hand-held non-contact polarized dermoscope (Dermlite, DL3 model, 3Gen, San Juan Capistrano, CA, USA). Serological work-up including routine blood test, thyroid function test (T3, T4, TSH), thyroid autoantibody (TG-Ab, TPO-Ab) (Chemiluminescence method, Abbott laboratories, Chicago, USA), IgE (Immune Turbidimetry, Siemens Healthcare Diagnostics, Marburg, Germany), antinuclear antibody (ANA), and dsDNA (ELISA, Aesku Diagnostics, Wendelsheim, Germany) were carried out for all diffuse AA and patchy AA patients. Besides, serum IgE level was evaluated also in 27 healthy subjects who did not have any hair loss and / or history of autoimmune diseases to serve as normal controls, among which, two individuals had history of atopic disease.
On the first visit, scalp biopsy specimens were taken from all 17 diffuse AA patients and 37 patchy AA patients from an active hair-loss area. The present study was approved by the ethics committee of our hospital and adhered to the tenets of the Declaration of Helsinki. Additionally, the written informed consents for the clinical photography, serological test, and biopsy were obtained from all the patients. Biopsy samples were divided into two parts for horizontal and vertical sections, fixed in 10% formalin, paraffin-embedded, and routinely processed for hematoxylin-eosin. Immunohistochemical staining of CD3 (DAKO 1:50), CD4 (DAKO 1:25), and CD8 (DAKO 1:50) were also performed in all diffuse AA cases. Perifollicular mononuclear cell, eosinophilic cell, CD3 + T cell, CD4 + T cell, and CD8 + T cell counts were done by two individuals using light microscopy from five fields of view at high-power (×400) magnification and values obtained were averaged.
Statistical analysis
Collected data were analyzed using SPSS version 13.0. Univariate analysis was performed using Fisher′s exact test and Wilcoxon rank sum test. Associations between inflammatory cells infiltration and laboratory values were analyzed using Spearman rank correlation test. A P value of <0.05 was considered statistically significant.
Results
Clinical findings
The study group comprised of eleven female and six male patients, aged 13 to 43 years with mean age of 27 years. Duration of hair loss ranged from ten days to six months with an average of 1.77 months. All of them presented in spring or summer complaining of an acute onset of diffuse hair loss for past few months/weeks. Sixteen patients had positive hair-pull test at their first visit. Nine of them had scalp pruritus before the onset of hair loss. Nine of them lost more than 80% of scalp hair within four months. Hair shedding was diffuse and rapid but hair regrowth started before shedding of entire scalp hair, and full recovery took place within six months [Figure - 1].
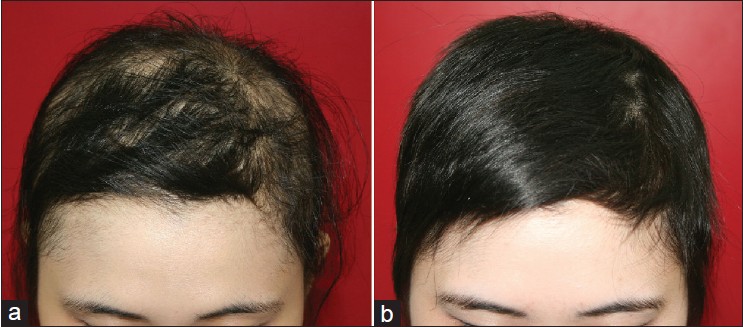 |
| Figure 1: Clinical pictures of diffuse alopecia areata patient (a) Diffuse hair thinning is seen with simultaneous hair regrowth; (b) Full recovery took place within 6 months |
Clinical characteristics of the 17 diffuse AA patients are summarized in [Table - 1]. Of the 17 diffuse AA patients, four had nail aberrations, two had a family history of AA, and three had a history of atopy manifesting as atopic dermatitis or/and allergic rhinitis.

Clinical comparison of the 37 patchy AA patients and 17 diffuse AA patients are summarized in [Table - 2]. Hair loss area was more extensive in diffuse AA than patchy AA patients in spite of similar duration (P<0.001). The incidence of positive hair-pull test and scalp pruritus before hair loss in diffuse AA were significantly higher than patchy AA (P = 0.008, 0.008, respectively). However, no statistical difference was found in the incidence of nail aberrations, atopic disease, and family history between the two groups.

Serological tests
Seven of 17 (41.2%) diffuse AA patients had increased serum IgE level (≥120 kU/l), and the incidence of its increase was higher than healthy control group (11.1%) (P = 0.027). Only one diffuse AA patient who had increased IgE level had ever suffered from atopic disease [Table - 3]. One patient with diffuse AA had elevated ANA. None of the patients had higher thyroid autoantibodies or eosinophilia. However, there was no statistical difference between diffuse AA and patchy AA group in respect to the above mentioned serological findings including serum IgE level.

Dermoscopic signs
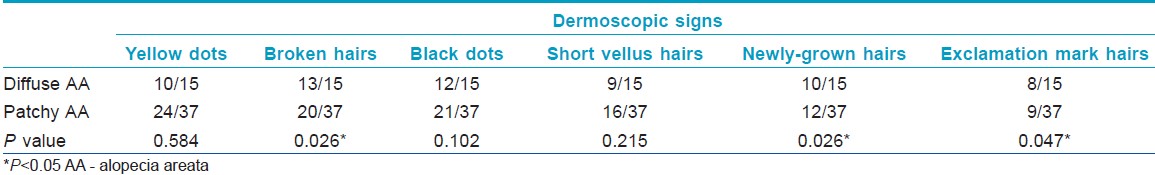
Dermoscopic signs found in the study group were characteristic of AA. Among the 15 diffuse AA patients examined, broken hairs, black dots, yellow dots, newly-grown short hairs, short vellus hairs, and exclamation mark hairs were seen in 13, 12, 10, 10, 9, and 8 cases, respectively. Furthermore, at least three of the above mentioned dermoscopic signs were found in each patient [Figure - 2]. The incidences of broken hairs, exclamation mark hairs, and newly grown short hairs were higher in diffuse AA patients than in patchy AA group (P = 0.026, 0.047, 0.026, respectively) [Table - 4].
 |
| Figure 2: Dermoscopic signs of diffuse alopecia areata (AA) patients Yellow dots, broken hairs, newly-grown short hairs in the same region of a diffuse AA patient |
Histopathological and immunohistochemical findings
Histopathology of scalp biopsies of diffuse AA showed typical features of AA which consisted of increased proportion of catagen and telogen hair follicles, and perifollicular inflammatory infiltration at the bulbar area [Figure - 3]a. Moreover, slight perivascular inflammatory cell infiltration comprising mainly of mononuclear cells was also noted. No major change was found in the epidermis. Besides, eosinophilic infiltration around lower hair follicles was also found in eight patients (47.1%) [Figure - 3]b. Lesional eosinophilic count in diffuse AA patients around hair follicles was 4.53 ± 5.71 (mean ± SD), while in patchy AA it was 1.62 ± 4.27. Overall eosinophilic count and mononuclear cell count were more intense (P = 0.048, 0.016, respectively) in diffuse AA as compared to patchy AA.
 |
| Figure 3: Histopathology of biopsied scalp tissue from diffuse alopecia areata patients (a) Intense inflammatory infiltration around and inside the hair bulb. Horizontal section. (b) Prominent eosinophilic infiltration around lower hair follicles. Vertical section (H and E, ×100) |
Immunohistochemical staining revealed intense T cells infiltration around hair bulbs in diffuse AA [Figure - 4]. CD4 + and CD8 + T cells were equally present in the lymphocytic infiltrates (CD4/CD8 ratio of 1:1.1). In contrast, CD4/ CD8 ratio in patchy AA was 2.3:1, indicating more CD4 + T cell involvement (P = 0.005) in patchy AA.CD3 + and CD8 + T cells infiltration around hair follicles were also more intense (P = 0.013, <0.001, respectively) in diffuse AA in comparison to patchy AA [Figure - 5], whereas no statistical difference was found in CD4 + T cells infiltration between the two groups. Moreover, no significant difference was found in terminal to vellus hair ratio and anagen to telogen hair ratio between the two groups [Table - 5].
 |
| Figure 4: Immunohistochemical staining of biopsied scalp tissue from diffuse alopecia areata patients. CD3+ (a), CD4+ (b), and CD8+ (c) T cells infiltration around hair follicles (DAB staining; ×200) |
 |
| Figure 5: Comparison of lesional inflammatory cells between diffuse alopecia areata (AA) and patchy AA. Mononuclear cells and eosinophils, CD3+ and CD8+ T cell infiltration around hair follicles in lesions of diffuse AA were more intense than in those of patchy AA (*P<0.05) |

We also analyzed the associations among lesional inflammatory cells infiltrations in diffuse AA and patchy AA groups. In diffuse AA group, eosinophilic count was found to positively correlate with mononuclear, CD3 + , CD4 + , and CD8 + T cell count (r = 0.719, 0.687, 0.629, 0.570; P<0.05). Furthermore, serum IgE level in diffuse AA patients positively correlated with lesional mononuclear, eosinophilic, and CD8 + T cells counts (r = 0.495, 0.595, 0.576, P<0.05), but showed no associations with CD3 + and CD4 + T cells counts. In contrast, no statistical correlation was found among inflammatory cell infiltrations in the patchy AA group.
Discussion
According to previous reports, diffuse AA has an acute onset and causes diffuse severe hair thinning within a few months. [1],[2] Women are more commonly affected. [3] Our study group of diffuse AA comprised of eleven female and six male patients with an average age of 27 years, which is similar to previous reports. [2],[4],[5]
It is believed that AA is an organ-specific autoimmune disease, targeting anagen-stage follicles which leads to disruption of hair fiber growth. [6] The source of antigenic targets has been suggested to be hair follicle melanocytes, dermal papilla cells, and keratinocytes, but as yet there is no concrete proof of specific targeting of these cell types. [7] Given the variety of AA presentations, it could be that different degrees and patterns of hair loss correspond to different target antigens. Elston et al. [8] found that eosinophils were common in all stages of AA, and they proposed that the presence of eosinophils is a helpful diagnostic feature of AA. However, the role of eosinophils in the pathogenesis of AA is still unclear to date. We found a more intense local inflammatory infiltration including mononuclear cells, eosinophils, CD3 + and CD8 + T cells in diffuse AA than in patchy AA. Furthermore, lesional eosinophilic infiltration in diffuse AA patients positively correlated with infiltration by mononuclear cells and by T lymphocytes, but this phenomenon was not present in patchy AA group. Therefore, a relatively more intense local inflammatory infiltration may be a pathologic feature of diffuse AA. However, whether this finding may serve as a link in the determination of etiopathogenetic mechanism of this diffuse type of hair loss or it is merely a concomitant phenomenon is unknown.
Previous studies on severe combined immunodeficient (SCID) mice revealed that transfer of hair loss in the human scalp explant/SCID mouse system requires CD8 + T cells with participation of CD4 + T cells. [9] The CD4/CD8 ratio in untreated AA patients has previously been evaluated to be 4:1. [10] However, in this study, this ratio was 1:1.1 in diffuse AA patients, whereas in patchy AA, it was found to be 2.3:1. Besides, CD8 + T cells infiltration around hair follicles was more intense in diffuse AA patients than in patchy AA patients. CD8 + T cell, being cytotoxic, may induce the apoptosis of hair follicle. Therefore, we presume that the intense infiltration of T cells (especially CD8 + T cells) is probably associated with the fast progression of diffuse AA. However, no definite pathological feature was found with diffuse AA and the small size of samples in this study, we doubt if one can differentiate diffuse AA from patchy AA based purely upon the biopsy.
Rebora [11] proposed that the onset of diffuse type of AA might be triggered by the fact that a relatively large amount of scalp hair happen to be in the early anagen VI sub-phase of the hair cycle simultaneously. In the present study, most of diffuse AA patients presented in May-September complaining of hair loss which begun in spring and summer (April-August), suggesting that diffuse AA may be triggered by an increase in seasonal allergens. Scalp pruritus before hair shedding, increased serum IgE level, and prominent eosinophilic infiltration in hair loss region were found in nearly half of diffuse AA patients in this group. Moreover, serum IgE level in diffuse AA patients positively correlated with the intensity of mononuclear cells, eosinophilic, and CD8 + T cells infiltration. In addition, unlike other subtypes of AA, diffuse AA usually has a shorter disease course and a better prognosis. Therefore, hypersensitivity should be taken in consideration in the pathogenesis of diffuse AA. However, the sample size of diffuse AA in this study is not big enough to draw concrete conclusion. Our future work will be aimed at enlarge the sample size, and to evaluate serum IgE level of specific allergen in order to investigate more profoundly into the pathogenesis of diffuse AA.
Diffuse AA can be misdiagnosed as other diffuse alopecia because it lacks the characteristic patches of AA, such as telogen effluvium (TE) and androgenic alopecia (AGA) Dermoscopy and pathological examination are of great value in diagnostic work. All the dermoscopic and pathological features can be found in diffuse AA patients, such as broken hairs, black dots, and exclamation mark hairs and follicular intense infiltration by mononuclear cells. However, TE and AGA lack all these features and can be excluded by pathologic findings, i.e., a terminal/vellus ratio of <4:1 without peri-bulbar lymphocyte infiltration is diagnostic of AGA, whereas the ratio >7:1 suggests TE. [12]
In summary, we reported a group of 17 patients with diffuse AA characterized by an onset in spring and summer with a fast progression, increased serum IgE level, higher incidence of dermoscopic signs such as broken hairs, black dots, and exclamation mark hairs, follicular intense infiltration by mononuclear cells, T lymphocytes, and eosinophils in hair loss region. We propose that hypersensitivity may be involved in the pathogenesis of diffuse AA and the acute onset of hair loss may be related to a relatively more intense local inflammatory infiltration of hair loss region and an increase in serum IgE level.
| 1. |
Dinh QQ, Chong AH. A case of widespread non-pigmented hair regrowth in diffuse alopecia areata. Australas J Dermatol 2007;48:221-3.
[Google Scholar]
|
| 2. |
Lew BL, Shin MK, Sim WY. Acute diffuse and total alopecia: A new subtype of alopecia areata with a favorable prognosis. J Am Acad Dermatol 2009;60:85-93.
[Google Scholar]
|
| 3. |
Tosti A, Whiting D, Iorizzo M, Pazzaglia M, Misciali C, Vincenzi C, et al. The role of scalp dermoscopy in the diagnosis of alopecia areata incognita. J Am Acad Dermatol 2008;59:64-7.
[Google Scholar]
|
| 4. |
Sato-Kawamura M, Aiba S, Tagami H. Acute diffuse and total alopecia of the female scalp. A new subtype of diffuse alopecia areata that has a favorable prognosis. Dermatology 2002;205:367-73.
[Google Scholar]
|
| 5. |
Choi HJ, Ihm CW. Acute alopecia totalis. Acta Dermatovenerol Alp Panonica Adriat 2006;15:27-34.
[Google Scholar]
|
| 6. |
McElwee KJ, Tobin DJ, Bystryn JC, King LE Jr, Sundberg JP. Alopecia areata: an autoimmune disease? Exp Dermatol 1999;8:371-9.
[Google Scholar]
|
| 7. |
Gilhar A, Kalish RS. Alopecia areata: A tissue specific autoimmune disease of the hair follicle. Autoimmun Rev 2006;5:64-9.
[Google Scholar]
|
| 8. |
Elston DM, McCollough ML, Bergfeld WF, Liranzo MO, Heibel M. Eosinophils in fibrous tracts and near hair bulbs: A helpful diagnostic feature of alopecia areata. J Am Acad Dermatol 1997;37:101-6.
[Google Scholar]
|
| 9. |
Gilhar A, Ullmann Y, Berkutzki T, Assy B, Kalish RS. Autoimmune hair loss (alopecia areata) transferred by T lymphocytes to human scalp explants on SCID mice. J Clin Invest 1998;101:62-7.
[Google Scholar]
|
| 10. |
Herbst V, Zöller M, Kissling S, Wenzel E, Stutz N, Freyschmidt-Paul P. Diphenylcyclopropenone treatment of alopecia areata induces apoptosis of perifollicular lymphocytes. Eur J Dermatol 2006;16:537-42.
[Google Scholar]
|
| 11. |
Rebora A. Alopecia areata incognita: A hypothesis. Dermatologica 1987;174:214-8.
[Google Scholar]
|
| 12. |
Whiting DA. Diagnostic and predictive value of horizontal sections of scalp biopsy specimens in male pattern androgenetic alopecia. J Am Acad Dermatol 1993;28:755-63.
[Google Scholar]
|
Fulltext Views
20,060
PDF downloads
3,131





