Translate this page into:
Diffuse large B-cell lymphoma of the skin and testis: A diagnostic and staging dilemma
Corresponding author: Dr. Meera Mahalingam, 1400 VFW PKWY, West Roxbury, Massachusetts 02132, USA. me@meeramahalingam.com
-
Received: ,
Accepted: ,
How to cite this article: Oh KS, Prajapati S, Mahalingam M. Diffuse large B-cell lymphoma of the skin and testis: A diagnostic and staging dilemma. Indian J Dermatol Venereol Leprol 2023;89:79-81.
Sir,
We describe a 46-year-old man with diffuse large B-cell lymphoma at two distinct extranodal sites presenting two months apart, to highlight the diagnostic and staging challenges in extranodal diffuse large B-cell lymphoma with discrepant immunoprofiles.
A 46-year-old man presented with an erythematous nodule on the left cubital fossa for three months [Figure 1a] and an occasionally itchy, migratory, erythematous rash on the left flank of one year duration. [Figure 1b]. He also reported a left testicular swelling of six-month duration and denied experiencing fever or other B symptoms. His personal and family history was unremarkable.

- Erythematous nodule on the left cubital fossa
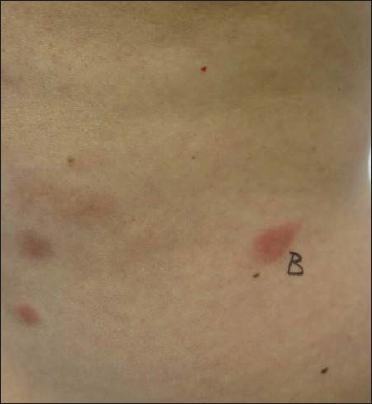
- Multiple erythematous plaques on the left flank
Two skin biopsies were performed (left flank and left elbow), both revealing a diffuse pan-dermal, non-epidermotropic infiltrate of confluent sheets of centroblasts and immunoblasts with frequent mitotic figures [Figure 2]. The neoplastic cells strongly expressed CD20, CD10 and PAX5 (negative CD43/Bcl-2/Bcl-6/MUM1/CD5/CD56/CD30). Based on this, the differential diagnosis considered was primary cutaneous diffuse large B-cell lymphoma, including primary cutaneous diffuse large B-cell lymphoma, leg type or a secondary cutaneous metastasis. A computerised tomography scan performed for staging purposes revealed multiple testicular lesions. A scrotal ultrasound showed two ill-defined focal areas of hypoechogenicity in the inferior aspect of the left testis [Figure 3]. Radical left orchiectomy revealed a diffuse large B-cell lymphoma [Figure 4]. Immunohistochemically, the neoplastic cells were diffusely CD20 and Bcl-2 positive and exhibited patchy positivity for CD10 and CD5 (negative Bcl-6/Bcl-1/MUM1). Fluorescence in situ hybridisation analysis of the testicular lymphoma revealed a t (8; 14) translocation, along with MYC rearrangement (negative for BCL6/BCL2 rearrangements). Flow cytometry analysis of the testicular mass showed a monotypic population of large B-cells that were CD19+/CD20+/CD10+/CD5dim+ and kappa light chain restricted.
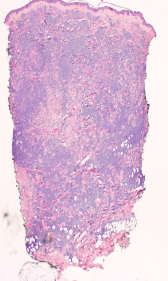
- Skin biopsy showing non-epidermotropic diffuse dermal lymphocytic infiltrates (H&E, ×40)
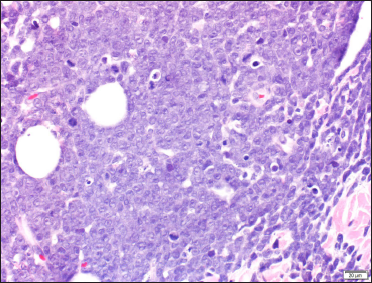
- High magnification of dermal lymphocytic infiltrates composed of closely packed centroblasts and immunoblasts (H&E, ×400)
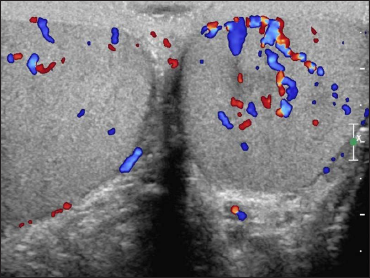
- Scrotal ultrasound: two ill-defined focal areas of hypoechogenicity in the inferior aspect of the left testis
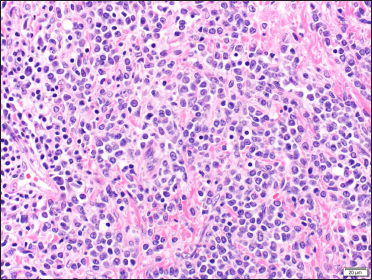
- Orchiectomy specimen showing sheets of centroblasts and immunoblasts (H&E, ×400)

- Neoplastic cells from orchiectomy specimen showing diffuse CD20 positivity (×100)
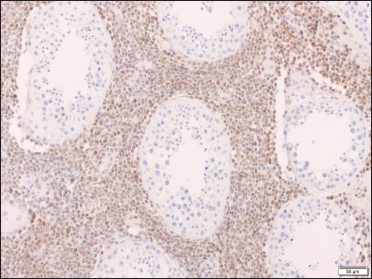
- Neoplastic cells from orchiectomy specimen showing diffuse Bcl-2 nuclear positivity (× 100)
His clinical staging remained indeterminate as it was unclear whether the cutaneous and testicular lymphoma were related or distinct. He subsequently received six cycles of rituximab/cyclophosphamide/hydroxydaunorubicin/oncovin, and prednisolone, along with three doses of prophylactic high-dose intrathecal methotrexate and irradiation of the contralateral testis. A repeat positron emission tomography/computed tomography scan performed upon completion revealed a complete response to therapy.
A year later, he presented with multiple purple-pink tender nodules on both arms. A punch biopsy of the nodules revealed histopathologic features similar to that noted in the first set of cutaneous biopsies. Immunohistochemically, however, and in contrast to the first set of cutaneous biopsies, the second set of cutaneous biopsies like the testicular biopsy were negative for CD10 but diffusely positive for Bcl-2. He was diagnosed with relapsed secondary diffuse large B-cell lymphoma and treated with second-line therapy (two cycles of rituximab/ifosfamide/carboplatin, and etoposide phosphate). A repeat positron emission tomography/computed tomography scan revealed a complete response to therapy. He was then treated with rituximab plus gemcitabine and oxaliplatin maintenance therapy.
Primary testicular lymphomas can spread to extra-nodal sites: including the contralateral testis, central nervous system, Waldeyer’s ring, lung, pleura, skin, and subcutaneous tissue.1 To date, there have been four reported cases of synchronous cutaneous and testicular lymphomas.2-5 Garcia Penalver et al. reported synchronous presentation of testicular swelling with skin and Waldeyer’s ring involvement by lymphoma. Although the morphology and immunohistochemical stains from the skin and Waldeyer’s ring were not reported, the testicular neoplastic cells were positive for CD20 and Bcl-2.2 Muniesa et al. reported three cases of primary cutaneous diffuse large B-cell lymphoma, leg type, two occurring simultaneously with a testicular B-cell lymphoma, while the third was a primary cutaneous diffuse large B-cell lymphoma, leg type with secondary lymphomatous involvement of the testis post-treatment.3 The skin lesions in all three cases were located on the legs and displayed strong positivity for Bcl-2, MUM-1 and FOXP1. These two cases were comparable to our case in terms of the synchronous presentation of cutaneous and testicular lesions. However, in contrast to our case, both the cutaneous as well as the testicular lymphoma exhibited similar morphology and immunoprofile. Similar to the third case in Muniesa et al. study, Grange et al. reported one case of primary cutaneous diffuse large B-cell lymphoma, leg type metastasizing to the testis, although the timeline and immunohistochemical staining profiles of both lesions were not reported and thus could not be compared.4 Dalal et al. reported a 40-year-old man with a rapidly enlarging and ulcerated abdominal mass and a testicular mass.5 Microscopic examination of both revealed a similar morphology and immunohistochemical profile (reported testicular neoplastic cells stained positive for CD45 and CD20, while negative for CD3/CD30/CD117) rendering a diagnosis of diffuse large B-cell lymphoma of the testis with secondary cutaneous involvement. Of note, Bcl-2 and MUM1 staining were not reported. Although lymphomas at both extra-nodal sites were diagnosed at the same time, the testicular mass preceded the presentation of the skin lesion by six months, suggesting a primary testicular lymphoma.
In conclusion, despite the similar cytomorphology, the discrepant immunophenotypic profile between the initially biopsied cutaneous lymphoma and the testicular lymphoma in our case was confounding for diagnostic and staging purposes. Testicular exploration may be considered for primary cutaneous diffuse large B-cell lymphoma, leg type to avoid overlooking the sub-clinical involvement of the testes.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflict of interest
There are no conflicts of interest.
References
- Primary testicular diffuse large B-cell lymphoma: A case report. World J Oncol. 2013;4:61-5.
- [CrossRef] [PubMed] [Google Scholar]
- Primary testicular lymphoma with extranodal involvement. Arch Esp Urol. 2009;62:489-93.
- [PubMed] [Google Scholar]
- Primary cutaneous diffuse large B-cell lymphoma, leg type and secondary cutaneous involvement by testicular B-cell lymphoma share identical clinicopathological and immunophenotypical features. J Am Acad Dermatol. 2012;66:650-4.
- [CrossRef] [PubMed] [Google Scholar]
- Primary cutaneous diffuse large B-cell lymphoma, leg type: Clinicopathologic features and prognostic analysis in 60 cases. Arch Dermatol. 2007;143:1144-50.
- [CrossRef] [PubMed] [Google Scholar]
- Primary testicular lymphoma with solitary cutaneous nodule as the initial presentation. Can Urol Assoc J. 2015;9:E744-7.
- [CrossRef] [PubMed] [Google Scholar]





